Abstract
Successful gene therapy of many genetic diseases requires efficient delivery of the gene to several tissues of the organism. Adeno‐associated virus (AAV) is, to date, the sole vehicle that allows to achieving this result but only at the condition of administering very large amounts of vectors. This, however, raises questions about the feasibility of the large‐scale production and about the safety of the approach. One way to overcome both problems would be to develop strategies that increase the in vivo efficiency. Here, we investigated the effect of fasting on the transduction efficiency of AAV serotypes 2, 6, and 9. The transgene expression was followed for several weeks and vector biodistribution was determined by real‐time polymerase chain reaction (PCR). The results show that fasting increases the transduction efficiency of all three serotypes. Altogether, we present here a simple and clinically acceptable approach that may allow to reducing the vector dose. Clin Trans Sci 2010; Volume 3: 333–336
Keywords: gene therapy, vector design and delivery, liver, muscle, viruses
Introduction
The successful treatment by gene therapy of neuromuscular disorders such as Duchenne muscular dystrophy requires efficient and body‐wide delivery of the therapeutic nucleic acids. Other genetic diseases such as hemophilia B require efficient production, by the liver or other tissues, of secreted factors. Currently, adeno‐associated viral vectors have the greatest potential for the treatment of such diseases. Notably however, efficient in vivo transduction requires the administration of large amounts of adeno‐associated virus (AAV) particles. 1 , 2 For example, between 1012 and 1013 vg of AAV‐6 have to be infused for obtaining widespread transduction of both cardiac and skeletal muscles in adult mice. 1 Based on this and other results, it is hypothesized that at least 1015 vg will have to be administered intravenously to obtain similar results in humans. Besides raising safety questions, the need of such amounts of viral vectors poses also technical problems. Indeed, for the production of such enormous amounts of AAV, one has first to develop a scalable and economical production method. If we take the routine technique employed to produce AAV in research laboratories, i.e., transient transfection of human embryonic kidney 293 cells in 15‐cm‐diameter tissue culture plates, production of 1015 particles would require 5,700 plates (1,000,000 cm2). 3 Although better large‐scale production systems become slowly available—in particular production in bioreactors using suspension cells—it still remains a challenge to produce such huge amounts of AAV vectors. The best way to overcome the safety and production problems would be to further increase the transduction efficiency of the AAVs. This may be achieved by modifying the capsid of the particles using genetic approaches. 4 , 5 , 6 Alternatively, some nongenetic strategies have also been explored as for example incorporation of AAV‐2 in a calcium phosphate coprecipitate. 7
In the present study, we were interested in evaluating the effect of fasting on the transduction efficiency of three different AAV serotypes, namely AAV‐2, ‐6, and ‐9. The rationale of the study was that fasting modifies the levels of lipids, sugars, and proteins in blood. This in turn could modify the concentration of factors that interact with AAV particles after intravenous injection. On the other hand fasting may induce cellular stress, which could increase cell uptake and/or modify the intracellular routing of the virus.
Methods
Recombinant AAV vector production
AAV vectors were produced in an adenovirus‐free system by triple transfection using polyethylenimine (PEI 25 kDa; Sigma‐Aldrich, Saint‐Quentin‐Fallavier, France) as described previously. 8 Briefly, human embryonic kidney 293 cells were transfected in 15 cm plates with the transcomplementing adenovirus helper plasmid pXX6, 9 the packaging plasmid expressing AAV‐2, ‐6, or 9‐ rep and cap genes, and the cis acting AAV vector plasmid pCMV‐mSeAP that contains the cDNA of a murine secreted alkaline phosphatase (mSeAP) subcloned into an AAV plasmid backbone which has the inverted terminal repeats (ITRs) of AAV‐2. The resulting AAV vector plasmids are under the transcriptional control of the CMV immediate‐early promoter and the SV40 polyA sequence. After 72 hours of transfection the cells underwent four cycles of freeze/thaw, the crude lysate was then treated with 25 U/ml benzonase, and AAV vectors were precipitated with cold saturated ammonium sulfate before purification by double CsCl2 ultracentrifugation gradient. This step was followed by extensive dialysis against sterile phosphate‐buffered saline containing Ca2+ and Mg2+. The concentration of encapsidated viral genomes was determined by real‐time quantitative PCR against a standard plasmid range and titers were expressed as viral genomes per ml (vg/ml).
In vivo experiments
Care and manipulation of mice were performed in accordance with national and European legislations on animal experimentation. Eight‐ to 10‐week‐old female Balb/C mice (Charles River, l’Arbresle, France) that have or not fasted for 18 hours (with free access to water) were injected into the tail vein with AAV particles in a final volume of 450 μL of sterile phosphate‐buffered saline supplemented with Ca2+ and Mg2+. Retro‐orbital plexus blood samples were obtained from anesthetized mice the day before the injection and thereafter every week using heparinized capillary tubes. Plasma was obtained by centrifugation at 4,000 g for 15 minutes and either analyzed immediately or stored at –20°C. Mice were sacrificed by cervical dislocation. Several tissues were collected and frozen in liquid nitrogen‐cooled isopentane.
Blood analysis
Endogenous alkaline phosphatase was heat‐inactivated for 5 minutes at 65°C and the heat‐resistant mSeAP was quantified by addition of the reaction buffer and CSPD chemiluminescent substrate (disodium 2‐chloro‐5‐(4‐methoxyspiro (1,2‐dioxetane‐3,2′‐(5′‐chloro)‐tricyclo (3.3.1.13,7)decan)‐4‐yl)‐1‐phenyl phosphate), according to the manufacturer’s instructions (Phosphalight kit TROPIX, Applera, Villebon sur Yvette, France). Chemiluminescence was measured in 96‐well plate format with a luminometer (Victor 2 1420 Multilabel counter, Perkin Elmer, Courtaboeuf, France). Expression levels were determined using a standard curve of purified human placental alkaline phosphatase and expressed as nanograms of mSeAP per ml of plasma.
DNA isolation and real‐time PCR
DNA isolation from tissues was performed using the Wizard Genomic DNA Purification Kit (Promega, Charbonnières‐les‐Bains, France) in accordance with the manufacturer’s protocol. Total DNA concentration was determined using a Nanodrop ND‐8000 spectrophotometer (Nanodrop Technologies, France), and 70 ng of DNA of each sample was used as the template material for real‐time‐PCR. Taqman real‐time PCR was performed on each sample for both the CMV promoter in order to determine copies of the viral genome, and the mouse titin gene, to standardize for number of mouse genomes present in each sample. Primers and probe used for CMV amplification were: 5′‐CATCAATGGGCGTGGATAGC‐3′ (forward), 5′‐GGAGTTGTTACGACATTTTGGAAA‐3′ (reverse) and 5′‐ATTTCCAAGTCTCCACCC‐3′ (probe). Primers and probe used for titin were: 5′‐AAAACGAGCAGTGACGTGAGC‐3′ (forward), 5′‐TTCAGTCATGCTGCTAGCGC‐3′ (reverse), and 5′‐TGCACGGAAGCGTCTCGTCTCAGTC‐3′ (probe). The PCR amplifications were performed using 70 ng of DNA diluted in Absolute QPCR ROX Mix (Thermo Fischer Scientific, Illkirch, France), 0.1 μM of Taqman probes, and 0.2 μM primers (forward and reverse) in a final volume of 18 μL. Cycling conditions consisted of a thermo‐start DNA polymerase activation step at 95°C for 15 minutes followed by 40 cycles of two steps, 15 seconds of denaturation at 95°C and 60 seconds of annealing at 60°C. The PCR was performed on a 7900 HT thermocycler (Applied Biosystem, Villebon sur Yvette, France). A standard dilution range of a plasmid containing CMV and titin sequences was used on each real‐time‐PCR plate as copy number control. All samples and controls were run in duplicate. Data are expressed as the number of viral genome copies per diploid genome (copies/nucleus).
Results and Discussion
We selected serotypes AAV‐2, ‐6, and ‐9 for studying the effect of fasting on the in vivo transduction efficiency. The three vectors contain a reporter gene driven by the ubiquitous and strong viral CMV immediate‐early promoter/enhancer flanked by AAV‐2 inverted terminal repeat sequences. To follow the overall in vivo transduction efficiency, we used as reporter gene a murine secreted alkaline phosphatase (mSeAP). mSeAP expression results in the production of a nonimmunogenic protein that allows specific detection in blood and tissues. Notably, the background is very low due to a higher thermostability of mSeAP as compared to the endogenous phosphatases. 10
Eight to 10‐week‐old female Balb/C mice that have or not fasted for 18 hours were injected into the tail vein with 1 × 1011 vg AAV‐2/animal and 2 × 1011 vg/animal for AAV‐6 and ‐9. The kinetic of secretion of the reporter protein was followed over a period of 35–42 days using the blood samples which were taken once per week. The mSeAP levels in plasma were quantified by chemiluminescent detection of the enzyme activity and the expression are expressed as nanograms of mSeAP per ml of plasma. Among the three serotypes, the strongest expression in the absence of treatment was observed with AAV‐9, with mSeAP expression peaking at day 35 at 360 ng/ml. This is in agreement with the findings of a comparative study of AAV serotypes 1–9, which showed that AAV‐9 injection resulted in the highest protein levels. 11 Also in agreement with literature, intravenous injection of AAV‐2 and ‐6 results in relatively close expression levels. 12 Most interestingly, as shown in Figure 1 , fasting increased in a significant manner the levels of mSeAP in blood with all three serotypes. In average, the increase was of 95%, 102% and 29% for AAV‐2, ‐6, and ‐9, respectively.
Figure 1.
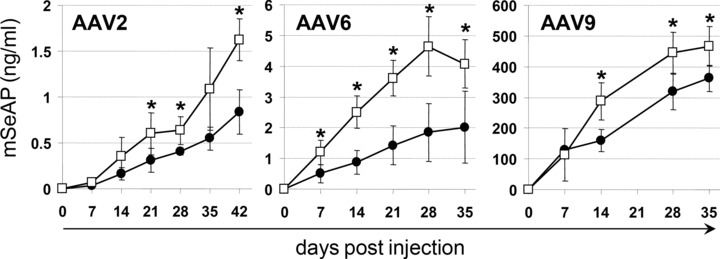
Influence of fasting on reporter gene expression. Mice fasted (empty squares) or not (filled circles) for 18 hours before infusion of the AAV. For AAV‐2, five animals per group were used and the vector dose that was injected was 1 × 1011 vg/animal. For AAV‐6 and ‐9, n= 4 and the injected vector dose was 2 × 1011 vg. All data are expressed as means ± standard deviation (SD). Differences between two groups were tested by using the unpaired t‐test with Welch correction as SDs could be different between the two populations tested. Statistical significance was defined by a two‐tailed p‐value below 0.05.
To evaluate whether the increased expression levels after fasting results from a more efficient cell uptake, we extracted genomic DNA from liver, heart, quadriceps, and diaphragm from control and treated mice. The vector genomes were then quantified in identical amounts of DNA using quantitative PCR ( Figure 2 ). AAV vector DNA was detected in all tissues examined, but copy numbers of recombinant genomes per cell varied significantly between the samples. As already reported for the three serotypes, most vector genomes were detected in the liver. 11 , 12 In agreement with the mSeAP plasma levels, genome numbers in the tissues were higher for AAV‐9 than those found for AAV‐2 and 6. Interestingly, we can note than fasting increases the vector genome number in all tissues for AAV‐9. The same trend, although not in all samples, was seen with the two other serotypes ( Figure 2 ).
Figure 2.
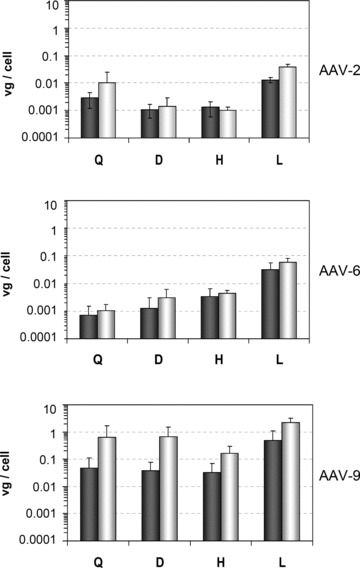
AAV vector biodistribution. The vector tissue biodistribution of AAV‐2, ‐6, and ‐9 in mice was analyzed by real‐time PCR in genomic DNA extracted from different muscles and from the liver. Results in quadriceps (Q), diaphragm (D), heart (H), and liver (L) are shown for control mice (gray bars) and mice that fasted for 18 hours (white bars). All data are expressed as means ± standard deviation; For AAV‐2, n= 3 and for AAV‐6 and 9, n= 4.
Conclusion
Our results show that fasting allows for an increased in vivo transduction efficiency of the three serotypes that have been evaluated. Although the enhancement that we observed was of at most twofold, one has to mention that there may be the possibility to further increase the effect by optimizing different parameters, in particular the duration of fasting and the injected vector dose. Notably, as alternative to short‐term fasting it could be interesting to investigate the influence of different types of low calorie diet on rAAV gene transfer efficiency. It is also worth mentioning that greater enhancements with fasting may be obtained by using other serotypes of AAV than those that were tested in the present study. Lastly, and most importantly, it remains to be shown whether our observation is restricted to mice or if it is of broader relevance—i.e., a similar or even greater enhancements can be obtained in larger animals.
How exactly fasting increases the AAV transduction efficiency remains unknown. Among the possible mechanisms that could be involved, we can cite the followings: (1) it is known that fasting reduces the content of circulating proteins, sugars, and cholesterol. 13 , 14 , 15 One could thus make the hypothesis that a decrease of the concentration of factors that interact with AAV particles after intravenous injection could lead to an enhanced availability of the viral particles and this, in turn, results in an increased transduction efficiency. (2) Fasting may induce cellular modifications which could increase the viral cell uptake (following either over‐expression of its receptor(s) or an increased endocytic activity) and/or modify the intracellular routing of the virus. For example, fasting induces autophagy, 16 a mechanism that is used by some viruses to increase their replication. 17 Whether recombinant AAVs can utilize the autophagic process remains, however, unknown.
Finally, we would like to underscore that although the enhancement remains of at most twofold, and that such as factor could be considered as negligible in the context of lab research, this is by far not the case when considering systemic administration of AAV for the treatment of human genetic disorders. Indeed, from a safety point of view (i.e., immune response against vector and/or the transgene and insertional mutagenesis) dividing by two the amounts of injected AAV particles would be a significant improvement. Also of importance, reducing by twofold the required amount of AAV would reduce the GMP production costs—an important step for gene therapy to become an acceptable medical treatment. Lastly, one has to underscore that fasting is not only a simple but also a clinically acceptable approach, even for patients affected by severe genetic disorders.
Acknowledgments
We thank Karine Poulard for advice on AAV production. We also thank Angélique Duvallet, Séverine Charles, Laetitia van Wittenberghe, and Béatrice Marolleau for animal experiments.
References
- 1. Gregorevic P, Blankinship MJ, Allen JM, Crawford RW, Meuse L, Miller DG, Russell DW, Chamberlain JS. Systemic delivery of genes to striated muscles using adeno‐associated viral vectors. Nat Med. 2004; 10: 828–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhu T, Zhou L, Mori S, Wang Z, McTiernan CF, Qiao C, Chen C, Wang DW, Li J, Xiao X. Sustained whole‐body functional rescue in congestive heart failure and muscular dystrophy hamsters by systemic gene transfer. Circulation. 2005; 112: 2650–2659. [DOI] [PubMed] [Google Scholar]
- 3. Cecchini S, Negrete A, Kotin RM. Toward exascale production of recombinant adeno‐associated virus for gene transfer applications. Gene Ther. 2008; 15: 823–830. [DOI] [PubMed] [Google Scholar]
- 4. Boucas J, Lux K, Huber A, Schievenbusch S, von Freyend MJ, Perabo L, Quadt‐Humme S, Odenthal M, Hallek M, Buning H. Engineering adeno‐associated virus serotype 2‐based targeting vectors using a new insertion site‐position 453‐and single point mutations. J Gene Med. 2009; 11: 1103–1113. [DOI] [PubMed] [Google Scholar]
- 5. Yang L, Jiang J, Drouin LM, Agbandje‐McKenna M, Chen C, Qiao C, Pu D, Hu X, Wang DZ, Li J, Xiao X. A myocardium tropic adeno‐associated virus (AAV) evolved by DNA shuffling and in vivo selection. Proc Natl Acad Sci U S A. 2009; 106: 3946–3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yu CY, Yuan Z, Cao Z, Wang B, Qiao C, Li J, Xiao X. A muscle‐targeting peptide displayed on AAV2 improves muscle tropism on systemic delivery. Gene Ther. 2009; 16: 953–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Walters RW, Duan D, Engelhardt JF, Welsh MJ. Incorporation of adeno‐associated virus in a calcium phosphate coprecipitate improves gene transfer to airway epithelia in vitro and in vivo. J Virol. 2000; 74: 535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Douar AM, Poulard K, Stockholm D, Danos O. Intracellular trafficking of adeno‐associated virus vectors: routing to the late endosomal compartment and proteasome degradation. J Virol. 2001; 75: 1824–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xiao X, Li J, Samulski RJ. Production of high‐titer recombinant adeno‐associated virus vectors in the absence of helper adenovirus. J Virol. 1998; 72: 2224–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang M, Orsini C, Casanova D, Millan JL, Mahfoudi A, Thuillier V. MUSEAP, a novel reporter gene for the study of long‐term gene expression in immunocompetent mice. Gene. 2001; 279: 99–108. [DOI] [PubMed] [Google Scholar]
- 11. Zincarelli C, Soltys S, Rengo G, Rabinowitz JE. Analysis of AAV serotypes 1–9 mediated gene expression and tropism in mice after systemic injection. Mol Ther. 2008; 16: 1073–1080. [DOI] [PubMed] [Google Scholar]
- 12. Grimm D, Zhou S, Nakai H, Thomas CE, Storm TA, Fuess S, Matsushita T, Allen J, Surosky R, Lochrie M, Meuse L, McClelland A, Colosi P, Kay MA. Preclinical in vivo evaluation of pseudotyped adeno‐associated virus vectors for liver gene therapy. Blood. 2003; 102: 2412–2419. [DOI] [PubMed] [Google Scholar]
- 13. Lee C, Safdie FM, Raffaghello L, Wei M, Madia F, Parrella E, Hwang D, Cohen P, Bianchi G, Longo VD. Reduced levels of IGF‐I mediate differential protection of normal and cancer cells in response to fasting and improve chemotherapeutic index. Cancer Res. 2010; 70: 1564–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cianflone K, Kalant D, Marliss EB, Gougeon R, Sniderman AD. Response of plasma ASP to a prolonged fast. Int J Obes Relat Metab Disord. 1995; 19: 604–609. [PubMed] [Google Scholar]
- 15. Volek JS, Sharman MJ, Gómez AL, DiPasquale C, Roti M, Pumerantz A, Kraemer WJ. Comparison of a very low‐carbohydrate and low‐fat diet on fasting lipids, LDL subclasses, insulin resistance, and postprandial lipemic responses in overweight women. Am Coll Nutr. 2004; 23: 177–184. [DOI] [PubMed] [Google Scholar]
- 16. Finn PF, Dice JF. Proteolytic and lipolytic responses to starvation. Nutrition. 2006; 22: 830–844. [DOI] [PubMed] [Google Scholar]
- 17. Kudchodkar SB, Levine B. Viruses and autophagy. Rev Med Virol. 2009; 19: 359–378. [DOI] [PMC free article] [PubMed] [Google Scholar]


