Figure 1.
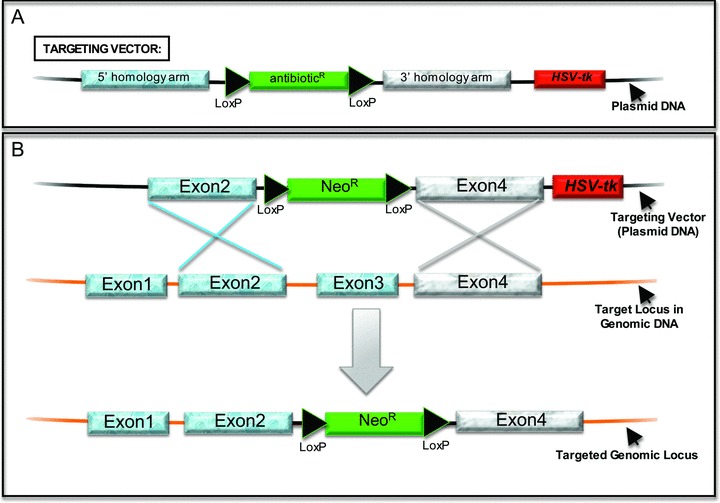
Model for gene targeting using homologous recombination. (A) This diagram of a targeting vector highlights its basic essential components: (1) an antibiotic (positive) selectable marker flanked by LoxP sites and located between 5′ and 3′ homology arms, and (2) a negative selection marker located outside the two homology arms, in this example the herpes simplex virus‐thymidine kinase (HSV‐tk) gene expressed by a constitutive promoter. The example provided is for disrupting a gene of interest (a “knockout”). The antibiotic expression cassette contains its own promoter in most targeting vectors but can be promoterless if targeting a gene expressed in the undifferentiated hESC. The latter approach requires a design that ensures that the endogenous promoter will express the antibiotic resistance gene. Targeting vectors can also be designed to introduce a reporter gene that will be under the control of the endogenous regulatory elements, in which case the reporter cDNA is located between the homology arms but outside of the LoxP‐flanked antibiotic selection cassette. The positive and negative selection cassettes can be oriented in either direction except when the positive selection cDNA is promoterless. Flp recombinase and Frt sites can be used similarly to Cre recombinase and LoxP sites. (B) This diagram outlines the process of homologous recombination (HR) using a targeting vector (top) designed to remove Exon3 of a target locus. The crossed lines indicate the crossing over event between the targeting vector and the genomic locus, which is mediated by host DNA repair machinery and requires homologous sequences. HR introduces the positive selectable (antibiotic) marker in place of Exon3. The negative selection marker, HSV‐tk in this example, will not be incorporated into the target DNA in cells that have properly undergone HR, allowing gancyclovir resistance to be used to select against recombination events that occurred through mechanisms other than HR. The LoxP‐flanked NeoR expression cassette can then be removed by expressing Cre recombinase, which can be introduced either by transient transfection of a Cre expression plasmid or by using Tat‐Cre protein.
