Abstract
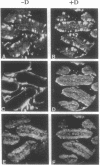
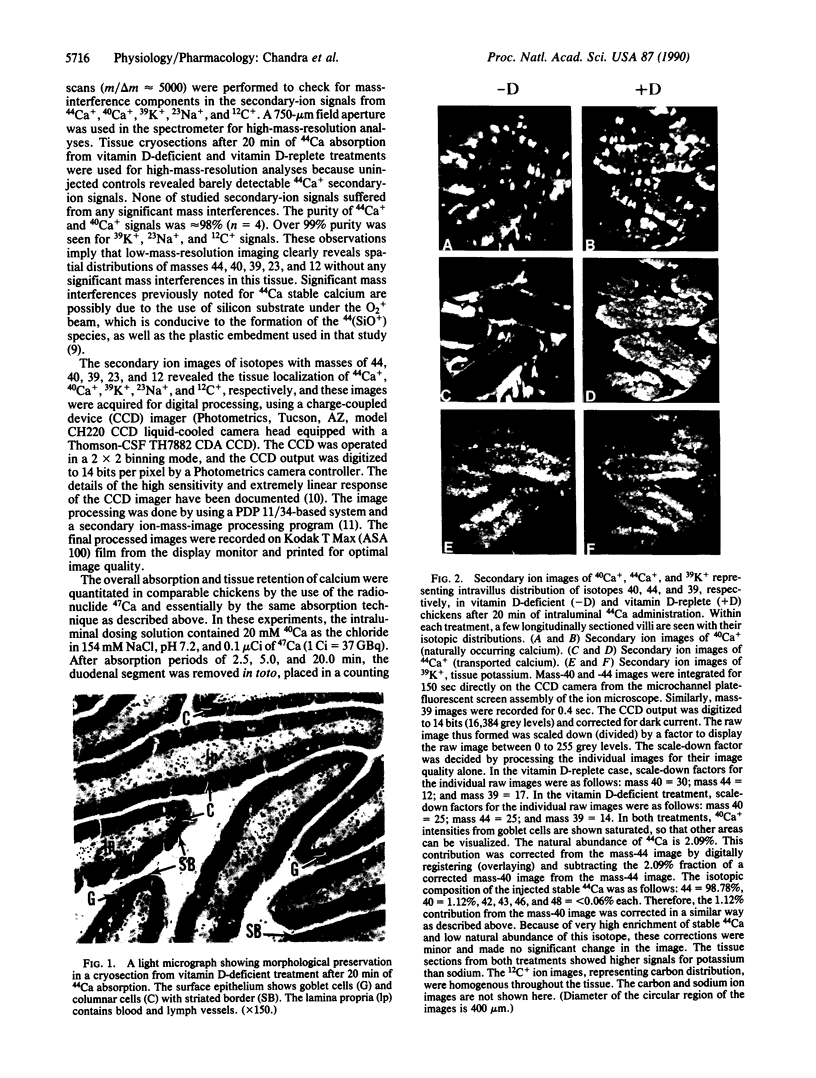
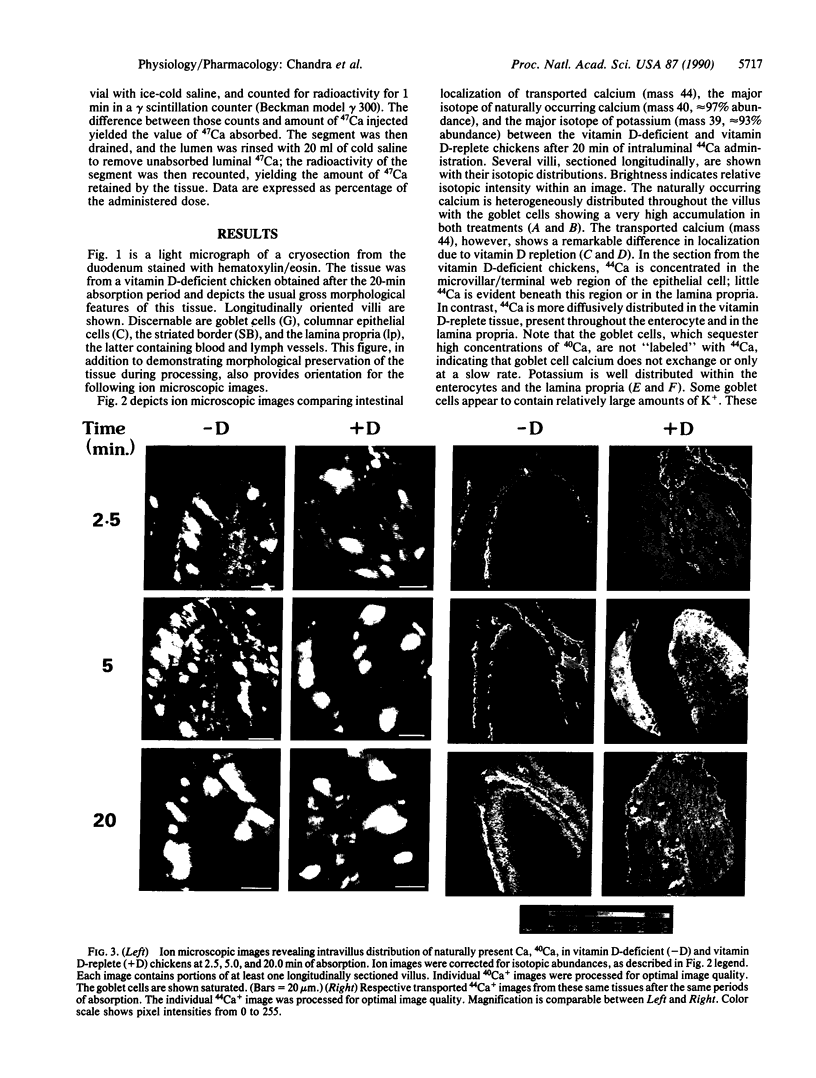
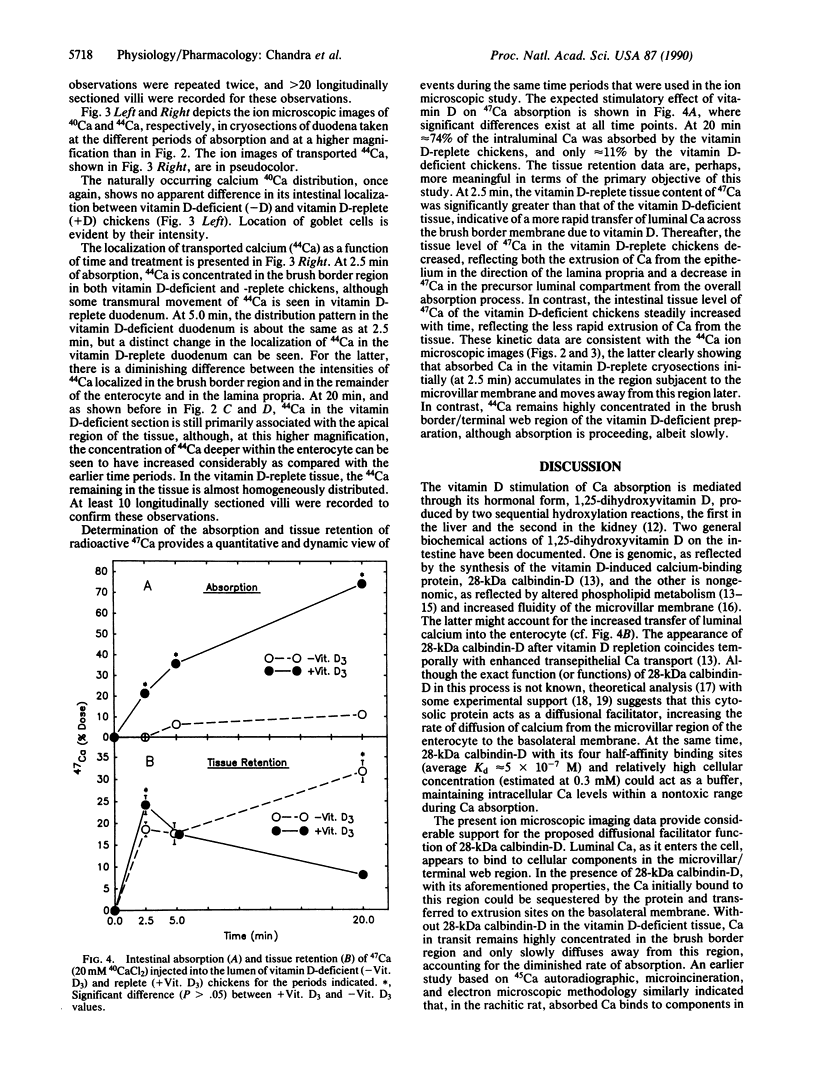
The intestinal absorption of calcium includes at least three definable steps; transfer across the microvillar membrane, movement through the cytosolic compartment, and energy-dependent extrusion into the lamina propria, Tracing the movement of calcium through the epithelium has been hampered by lack of suitable techniques and, in this study, advantage was taken of ion microscopy in conjunction with cryosectioning and use of the stable isotope 44Ca to visualize calcium in transit during the absorptive process. The effect of vitamin D, required for optimal calcium absorption, was investigated. Twenty millimolar 44Ca was injected into the duodenal lumen in situ of vitamin D-deficient and vitamin D-replete chickens. At 2.5, 5.0, and 20.0 min after injection, duodenal tissue was obtained and processed for ion microscopic imaging. At 2.5 min. 44Ca was seen to be concentrated in the region subjacent to the microvillar membrane in tissue from both groups. At 5.0 and 20.0 min, a similar pattern of localization was evident in D-deficient tissues. In D-replete tissues, the distribution of 44Ca became more homogenous, indicating that vitamin D increased the rate of transfer of Ca2+ from the apical to the basolateral membrane, a function previously ascribed to the vitamin D-induced calcium-binding protein (28-kDa calbindin-D). Quantitative aspects of the calcium absorptive process were determined in parallel experiments with the radionuclide 47Ca. Complementary information on the localization of the naturally occurring isotopes of calcium (40Ca) and potassium (39K) is also described.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bikle D. D., Munson S., Chafouleas J. G. The role of calmodulin in 1,25-dihydroxyvitamin D regulation of calcium transport across the intestinal brush border membrane. Prog Clin Biol Res. 1984;168:193–198. [PubMed] [Google Scholar]
- Brasitus T. A., Dudeja P. K., Eby B., Lau K. Correction by 1-25-dihydroxycholecalciferol of the abnormal fluidity and lipid composition of enterocyte brush border membranes in vitamin D-deprived rats. J Biol Chem. 1986 Dec 15;261(35):16404–16409. [PubMed] [Google Scholar]
- Burns M. S. Selection of calcium isotopes for secondary ion mass spectrometric analysis of biological material. J Microsc. 1984 Aug;135(Pt 2):209–212. doi: 10.1111/j.1365-2818.1984.tb00521.x. [DOI] [PubMed] [Google Scholar]
- Chandra S., Morrison G. H. Ion microscopy in biology and medicine. Methods Enzymol. 1988;158:157–179. doi: 10.1016/0076-6879(88)58054-0. [DOI] [PubMed] [Google Scholar]
- Edelstein S., Fullmer C. S., Wasserman R. H. Gastrointestinal absorption of lead in chicks: involvement of the cholecalciferol endocrine system. J Nutr. 1984 Apr;114(4):692–700. doi: 10.1093/jn/114.4.692. [DOI] [PubMed] [Google Scholar]
- Feher J. J. Facilitated calcium diffusion by intestinal calcium-binding protein. Am J Physiol. 1983 Mar;244(3):C303–C307. doi: 10.1152/ajpcell.1983.244.3.C303. [DOI] [PubMed] [Google Scholar]
- Feher J. J., Fullmer C. S., Fritzsch G. K. Comparison of the enhanced steady-state diffusion of calcium by calbindin-D9K and calmodulin: possible importance in intestinal calcium absorption. Cell Calcium. 1989 May-Jun;10(4):189–203. doi: 10.1016/0143-4160(89)90002-x. [DOI] [PubMed] [Google Scholar]
- Hagler H. K., Lopez L. E., Flores J. S., Lundswick R. J., Buja L. M. Standards for quantitative energy dispersive X-ray microanalysis of biological cryosections: validation and application to studies of myocardium. J Microsc. 1983 Aug;131(Pt 2):221–234. doi: 10.1111/j.1365-2818.1983.tb04248.x. [DOI] [PubMed] [Google Scholar]
- Ling Y. C., Bernius M. T., Morrison G. H. SIMIPS: secondary ion mass image processing system. J Chem Inf Comput Sci. 1987 May;27(2):86–94. doi: 10.1021/ci00054a009. [DOI] [PubMed] [Google Scholar]
- Matsumoto T., Fontaine O., Rasmussen H. Effect of 1,25-dihydroxyvitamin D3 on phospholipid metabolism in chick duodenal mucosal cell. Relationship to its mechanism of action. J Biol Chem. 1981 Apr 10;256(7):3354–3360. [PubMed] [Google Scholar]
- Morrissey R. L., Wasserman R. H. Calcium absorption and calcium-binding protein in chicks on differing calcium and phosphorus intakes. Am J Physiol. 1971 May;220(5):1509–1515. doi: 10.1152/ajplegacy.1971.220.5.1509. [DOI] [PubMed] [Google Scholar]
- Nemere I., Leathers V., Norman A. W. 1,25-Dihydroxyvitamin D3-mediated intestinal calcium transport. Biochemical identification of lysosomes containing calcium and calcium-binding protein (calbindin-D28K). J Biol Chem. 1986 Dec 5;261(34):16106–16114. [PubMed] [Google Scholar]
- O'Doherty P. J. 1,25-Dihydroxyvitamin D3 increases the activity of the intestinal phosphatidylcholine deacylation-reacylation cycle. Lipids. 1979 Jan;14(1):75–77. doi: 10.1007/BF02533571. [DOI] [PubMed] [Google Scholar]
- Sampson H. W., Matthews J. L., Martin J. H., Kunin A. S. An electron microscopic localization of calcium in the small intestine of normal, rachitic, and vitamin-D-treated rats. Calcif Tissue Res. 1970;5(4):305–316. doi: 10.1007/BF02017560. [DOI] [PubMed] [Google Scholar]
- Wasserman R. H., Brindak M. E., Meyer S. A., Fullmer C. S. Evidence for multiple effects of vitamin D3 on calcium absorption: response of rachitic chicks, with or without partial vitamin D3 repletion, to 1,25-dihydroxyvitamin D3. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7939–7943. doi: 10.1073/pnas.79.24.7939. [DOI] [PMC free article] [PubMed] [Google Scholar]