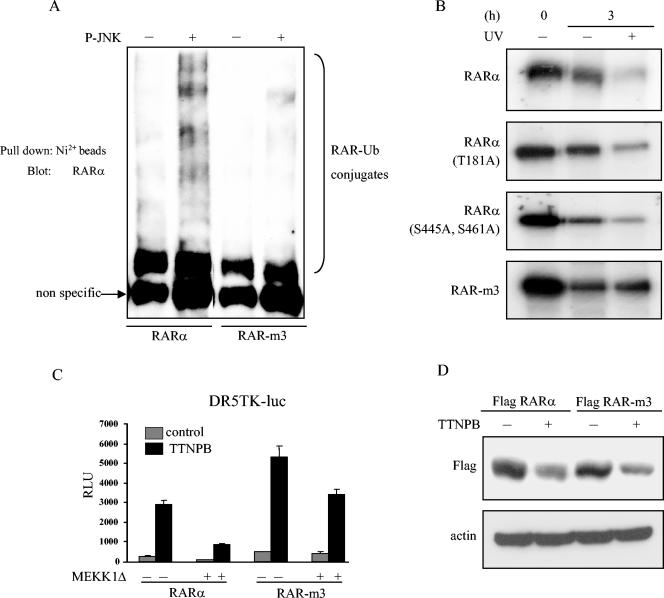
FIG. 7.
RAR-m3 is protected from JNK-mediated ubiquitination and degradation. (A) Mutant RARα is resistant to JNK-mediated ubiquitination. Wild-type RARα and RAR-m3 were subjected to kinase reactions with (+) or without (−) active JNK, followed by in vitro ubiquitination reactions. (B) Mutant RARα is resistant to UV-induced degradation. Pulse-chase experiments were carried out to measure the stability of wild-type and mutant RARα. HeLa cells were cotransfected with expression vectors containing JNK1 and wild-type or mutant RARα and labeled with [35S]methionine. Transfectants were treated or not treated with UV radiation at the start of the chase (t = 0). After 3 h, cells were lysed, and RARα was immunoprecipitated with anti-Flag antibodies and visualized by autoradiography. (C) Transfection of mutant RARα partially abrogates stress kinase-induced suppression of RARE activity. COS-1 cells were cotransfected with DR5TK-luc reporters and expression vectors containing MEKK1Δ, β-Gal, and wild-type RARα or RAR-m3. Transfectants were treated or not treated with 1 μM TTNPB and subjected to luciferase assays. Results were corrected for differences in transfection efficiency and expressed as the means ± standard deviations from three identical wells. (D) Mutant RARα undergoes degradation after ligand binding. HeLa cells were transiently transfected with expression vectors containing wild-type RARα or RAR-m3. After 24 h, cells were treated (+) or not treated (−) with TTNPB (10−6 M) and lysed, after which the lysates were subjected to Western blot analysis with anti-Flag antibodies.