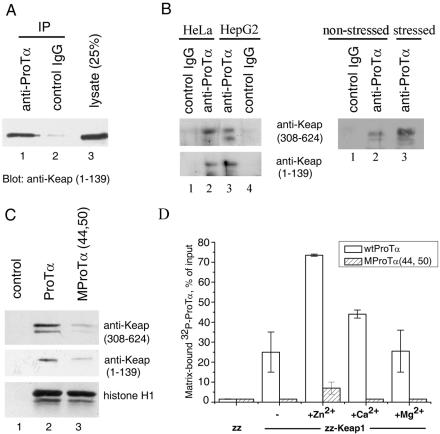
FIG. 2.
Keap1 interacts with ProTα in human cell lysates (A, B, and C) and in vitro (D). (A) Coimmunoprecipitation of the ectopically produced Keap1 with ProTα from a whole-cell lysate of HeLa cells. Precipitation was performed with anti-ProTα 2F11 monoclonal antibody (lane 1) or with control mouse IgG (lane 2). Lane 3, whole-cell lysate. Detection of Keap1 was performed by Western blot analysis with anti-Keap1 polyclonal antibodies. (B) Endogenous Keap1 associates with ProTα. Left panel: Coprecipitation of endogenous Keap1 with ProTα from whole-cell lysates of HeLa (lanes 1 and 2) and HepG2 (lanes 3 and 4) cells. Precipitation and detection were performed as described for (A). Right panel: Coprecipitation of endogenous Keap1 from lysates of HeLa cells either untreated (lanes 1 and 2) or treated with 100 μM DEM for 4 h (lane 3). Note that in panels A and B the membranes were exposed to X-ray film for 30 s and 30 min, respectively. (C) Binding of endogenous Keap1 to Sepharose-immobilized ProTα is impaired by the (44,50) mutation in ProTα. Lane 1, resin without ProTα; lane 2, wild-type ProTα; lane 3, MProTα(44,50) mutant. The amount of Keap1 from HeLa whole-cell lysates bound to each resin was evaluated as described for panel A with two different anti-Keap1 antibodies. Bottom row: histone H1 binding to the same affinity resins, verifying equal amounts of immobilized ProTα. (D) Interaction of the purified recombinant ProTα and Keap1 in vitro. IgG-Sepharose-immobilized zz-Keap1 (150 ng) or zz alone (30 ng) was incubated with 32P-radiolabeled wild-type (wt) ProTα or the MProTα(44,50) mutant, either in the absence or in the presence of the indicated metal cations (100 μM). After incubation, the unbound ProTα was removed by washing the resin, and the amount of 32P-ProTα bound to each affinity resin was determined by measuring the radioactivity. Error bars indicate standard deviations.