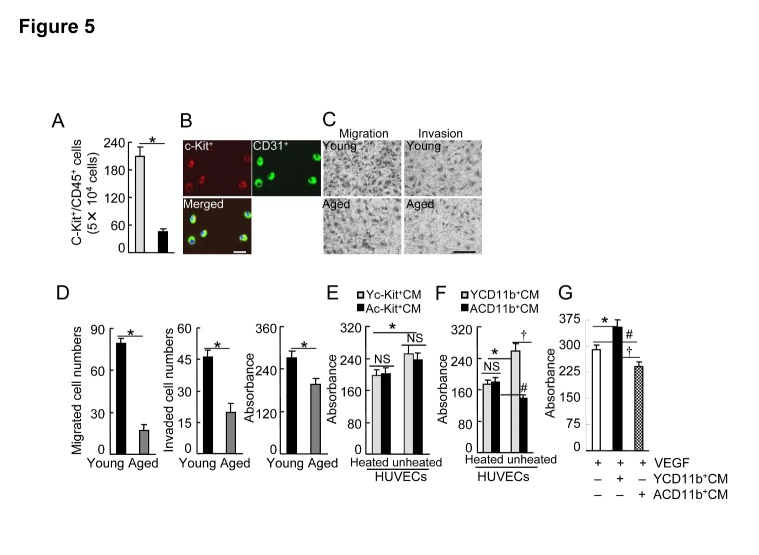
Figure 5. Effects of aging on BM EPC mobilization and cellular functions.
A) The flow cytometry analysis showed that the numbers of circulating c-Kit+/CD31+ cells were decreased in the aged mice compared to the young mice (n=6). B) Progenitor cell surface makers of (c-Kit and CD31) were expressed in BM-derived c-Kit+ cells cultured in EGM-2 for 4 days after isolation with magnetic beads. C and D) Representative images and combined quantitative data showing that aging impaired c-Kit+ cell migration, invasion, or/and proliferation (n=5-6). E and F) Yc-Kit+Cs or YCD11b+Cs and Ac-Kit+Cs or ACD11b+Cs were cultured (six-well-plates) in serum-free EBM-2 (for the former) or RPMI medium 1640 (for the later) under hypoxic condition (plates in hypoxic cambers) for 36 hr (for c-Kit+ cells) or 48 hr (CD11b+ cells), respectively. Following collection of the culture medium (Yc-Kit+CM, Ac-Kit+CM; YCD11b+CM, ACD11b+CM), the special fraction containing an approx. >70 kDa protein was isolated with 150 K and then 50 K AmiconUltra contricons, and we adjusted the protein concentration to 1.5 mg/ml for the cellular experiments. For the proliferation assays, 5 × 103 HUVECs were incubated in EBM-2 supplemented with either heated or unheated Yc-Kit+CM, Ac-Kit+CM (E), YCD11b+CM, or ACD11b+CM (F) (20 μl/100 μl EBM-2 for each), respectively, for 48 hr, and then were subjected to MTS assays. G) HUVECs were incubated with VEGF-A (50 ng/mL), YCD11b+CM, or ACD11b+CM (20 μl/100 μl EBM-2 for both) respectively for 48 hr, and then were subjected to an MTS assay. Data are mean ± SEM (n=5-6). *P<0.05, †P<0.05, #P<0.05 by one-way ANOVA and Tukey’s post hoc tests. Scale bar, 50 μm.