Abstract
Hepatic cirrhosis is often accompanied by functional kidney impairment, which may be reversed if early treatment is promptly administered. This study aimed to investigate the role of Cystatin C and Cystatin C estimated glomerular filtration rate in the diagnosis of kidney impairment in patients with hepatic cirrhosis.
Four hundred sixty five patients with hepatic cirrhosis were recruited. Serum creatinine and Cystatin C were determined, and their estimated glomerular filtration rates were calculated.
The area under the receiver-operating characteristic curve (area under curve [AUC]) of Cystatin C and Cystatin C estimated glomerular filtration rate was significantly larger than that of serum creatinine and serum creatinine estimated glomerular filtration rate, respectively (P = .000). When the optimal cut-off value and upper reference limit were used, similar sensitivity, misdiagnosis rate, and diagnostic consistency were only observed in Cystatin C estimated glomerular filtration rate (P > .05).
Cystatin C and Cystatin C estimated glomerular filtration rate are superior to serum creatinine and serum creatinine estimated glomerular filtration rate in diagnosis of secondary kidney impairment, and Cystatin C estimated glomerular filtration rate has a better performance as compared with Cystatin C. However, it is not a measured parameter, and thus the lab should determine its own optimal cut-off value.
Keywords: Cystatin C, Cystatin C estimated glomerular filtration rate, Hepatic cirrhosis
1. Introduction
In hepatic cirrhosis patients, portal hypertension usually causes the insufficiency of effective circulating volume and alters the hemodynamics, leading to re-distribution of blood flow in the kidney, water-sodium retention, and reduced glomerular filtration rate (GFR). Thus, hepatic cirrhosis is often accompanied by functional kidney impairment.[1] However, this impairment may be reversed if early treatment is promptly administered.[2] On the contrary, the continuation and/or progression of kidney impairment and altered hemodynamics may significantly increase the vasoactive substances such as renin-aldosterone, vasopressin, and endothelin and elevate sympathetic nervous system activity, finally leading to hepatorenal syndrome (HRS).[3] Acute kidney injury (AKI) will develop if it is serious enough,[4] which can often be life-threatening and lethal. Thus, accurate and early diagnosis of kidney impairment is of great importance in hepatic cirrhosis patients.
Enzyme assay developed in recent years for detection of serum creatine (SCr) detection, especially, has the advantages of high selectivity, high sensitivity, simple operation, and high anti-interference ability and has been widely used in clinical laboratories. According to the guideline of Kidney Disease Improving Global Outcomes (KDIGO), AKI and chronic kidney disease (CKD) are defined according to the SCr. However, it is well known that SCr as an important indicator reflecting kidney function still has severe limitations: its level is influenced by age, sex, muscle mass, and renal tubular excretion, and it has limited potential in the diagnosis of early kidney impairment. Thus, use of SCr in the early diagnosis of kidney impairment in hepatic cirrhosis patients has the possibility to cause missed diagnosis, which could lead to therapeutic failure in future treatment. In recent years, some studies have confirmed that Cystatin C (CysC) is superior to SCr in the diagnosis of kidney impairment.[5,6] The international organization on kidney diseases propose that it is necessary to calculate the estimated glomerular filtration rate (eGFR), but not a specific biochemical parameter, as a marker of kidney function.[7] In clinical practice, physicians should also use eGFR to evaluate the kidney function.[8] In the present study, the serum CysC concentration was measured in hepatic cirrhosis patients, and the eGFR was calculated on the basis of serum CysC (eGFRcysc). Finally, the value of eGFRcysc and SCr-based eGFR (eGFRscr) in the early diagnosis of secondary kidney impairment was compared in these patients.
2. Methods
2.1. Patients
A total of 465 patients with hepatic cirrhosis were recruited from Mianyang Central Hospital (Sichuan, China) between August 2012 and December 2014. There were 330 men and 135 women with the mean age of 56.2 ± 13.1 years (range: 19–89 years). Hepatic cirrhosis was diagnosed according to the guideline for hepatic fibrosis developed by the Asian Pacific Association for the Study of the Liver.[9] In addition, hepatitis B cirrhosis was found in 265 patients, hepatitis C cirrhosis in 41, alcoholic cirrhosis in 33, alcoholic combined hepatitis related cirrhosis in 62, autoimmune cirrhosis in 35, and cirrhosis of unknown cause in 29. Primary kidney disease, diabetic mellitus, cardiovascular dysfunction, and respiratory dysfunction were excluded from these patients. Vasoactive drugs or somatostatin were not used within 1 week before blood collection. Kidney impairment was determined according to the KDIDO Clinical Practice Guideline for Acute Kidney Injury or KDIDO 2012 Clinical Practice Guidelines for the Evaluation and Management of Chronic Kidney Disease.
The study protocol was approved by the Medical Ethics Committee of Mianyang Central Hospital (2012–08-AJ), and written informed consent was obtained from each patient before study. According to Child-Pugh classification and MELD scoring system,[10,11] patients were divided into 3 subgroups according to the severity of cirrhosis: Child-Pugh class A (Child A, n = 202), Child-Pugh B (Child B, n = 192), and Child-Pugh C (Child C, n = 71) groups.
2.2. Blood sampling
Venous blood was collected from each patient in the morning after being fasted overnight. Serum was separated by centrifugation at 3000 rpm for 15 minutes within 2 hours after sample collection. All the laboratory tests were completed within 8 hours. Sodium citrate anti-coagulated blood was centrifuged at 3000 rpm for 15 minutes, and the plasma was harvested for the detection of prothrombin time (PT) within 2 hours.
2.3. Child-Pugh classification-related indexes measurement
Serum total bilirubin and albumin were measured with the LabospectTM 008 fully automatic analyzer (Hitachi, Japan) and the kits were purchased from Sichuan Maccura Biotechnology Co., Ltd (Sichuan, China). PT was measured with the CS-5100 automated blood coagulation analyzer (Sysmex, Japan) and the kit was from Siemens Healthcare Diagnostics Inc (Newark, USA).
2.4. Serum creatinine and Cystatin C measurements
SCr and CysC were measured with the LabospectTM 008 fully automatic analyzer (Hitachi, Japan). The detection limits of CysC and SCr were 0.13∼7.80 mg/L and 7.1∼8840 μmol/L, respectively. Kits were purchased from Sichuan Maccura Biotechnology Co., Ltd (Sichuan, China), but CysC reagents were original equipment manufacture (OEM) products that were obtained from Gentian (Moss, Norway).
2.5. Calculation of estimated GFR
The eGFR formula developed in Chinese was used to calculate the eGFR (mL/min/1.73 m2). The eGFR formula of CysC was developed by our group using “true GFR” measured with 99mTc-DTPA and has been published elsewhere[12] (equation 1) and used to calculate the eGFRCysC.
The modification of diet in renal disease (MDRD) equation (c-aGFR)[13] was developed by a Chinese group (equation 2) and used to calculate eGFRSCr.
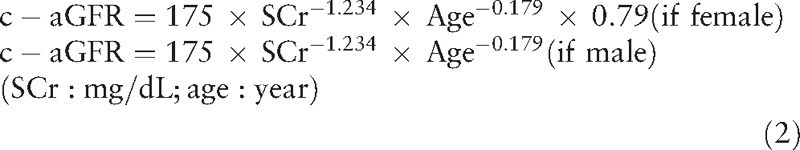 |
2.6. Statistical analysis
Quantitative data are expressed as mean ± standard deviation (SD) and median (min, max). Normal distribution was tested with Kolmogorov–Smirnov method and Q–Q chart. Quantitative data with normal distribution were compared with one-way analysis of variance (ANOVA), and those with abnormal distribution with Kruskal–Wallis non-parametric test. Qualitative data were tested with chi-squared test. The diagnostic efficiencies of SCr, CysC, and eGFR were analyzed with receiver operating characteristic (ROC) curve in which the area under curve (AUC) was tested with Delong non-parametric test. Kappa test was used to examine the consistency between tentative diagnosis and clinical diagnosis. The kappa coefficient of different parameters and at different cut-off values was compared with Fisher Z-transformation method. Statistical analysis was performed with SPSS 19.0 (SPSS, Inc., Somers, NY) and MedCalc11.5 (MedCalc Software, Mariakerke, Belguim). A value of P < .05 was considered statistically significant.
3. Results
3.1. Quality control
The low reference limit of CysC was 0.03 mg/L in the detection. The intra-assay coefficient of variation (CV) was 1.86% (mean, 0.79 mg/L; n = 20), and the day-to-day CV was 2.23% (mean, 0.46 mg/L; n = 30) and 2.44% (mean, 4.63 mg/L; n = 30).
The low reference limit of SCr was 2.40 mol/L in the detection. The intra-assay CV was 1.35% (mean, 83.9 μmol/L; n = 20), and the day-to-day CV was 2.37% (mean, 72.5 μmol/L; n = 30) and 1.21% (mean, 590.4 μmol/L; n = 30).
3.2. Characteristics of patients at baseline
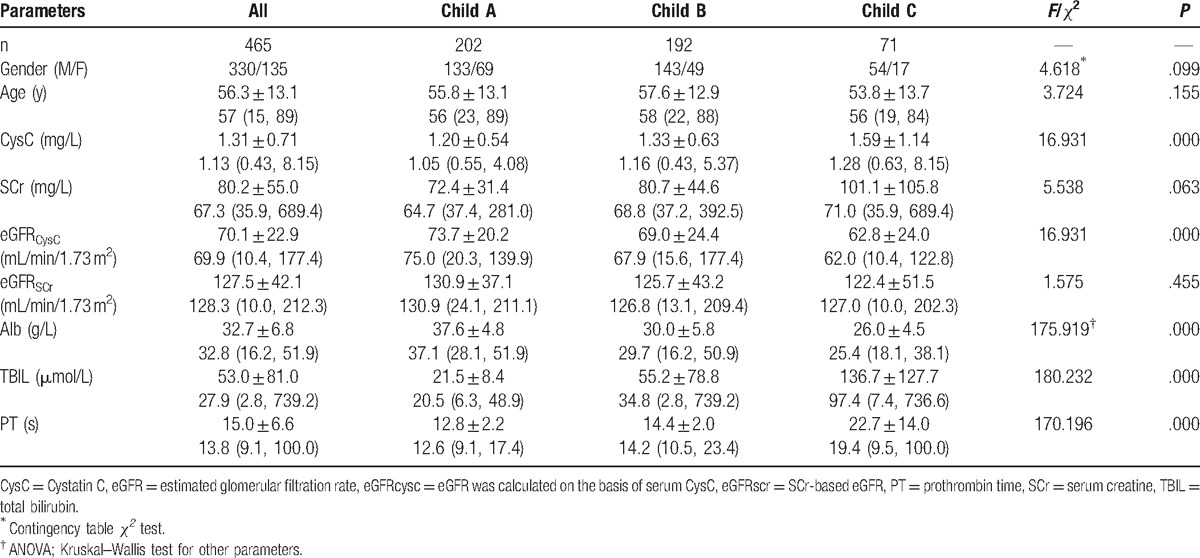
Of 465 patients with hepatic cirrhosis, the Child-Pugh classes and results from laboratory examinations are shown in Table 1. Normal distribution test with Kolmogorov–Smirnov method and Q–Q chart showed parameters except for Alb displayed abnormal distribution.
Table 1.
Characteristics of patients with liver cirrhosis at baseline.
3.3. Diagnostic efficiency of SCr, CysC, and eGFR for kidney impairment in patients with hepatic cirrhosis
ROC analysis was employed to analyze the diagnostic efficiencies of SCr, CysC, eGFRSCr, and eGFRCysC for kidney impairment in hepatic cirrhosis patients (Fig. 1). Results showed the optimal cut-off values of SCr (M/F), CysC, eGFRSCr, and eGFRCysC were 76.8/62.6 μmol/L (M/F), 1.24 mg/L, 109 mL/min/1.73 m2, and 63.4 mL/min/1.73 m2, respectively, in the diagnosis of kidney impairment (Table 2). Under these conditions, the highest sensitivity was found in CysC (87.6%) and lowest sensitivity in eGFRSCr (66.7%); the highest specificity was noted in eGFRCysC (94.4%) and the lowest specificity in SCr (M: 81.0%; F: 88.0%). The diagnostic efficiency was comparable between CysC and eGFRCysC (P > .05) and between SCr and eGFRSCr (P > .05). Further analysis with Delong non-parametric test was performed for the AUC of different parameters (Table 3). Results showed the AUCs of CysC and eGFRCysC (AUC-CysC and AUC-eGFRCysC) were significantly higher than those of SCr and eGFRSCr (AUC-SCr and AUC-eGFRSCr) (P < .05) (Table 3).
Figure 1.
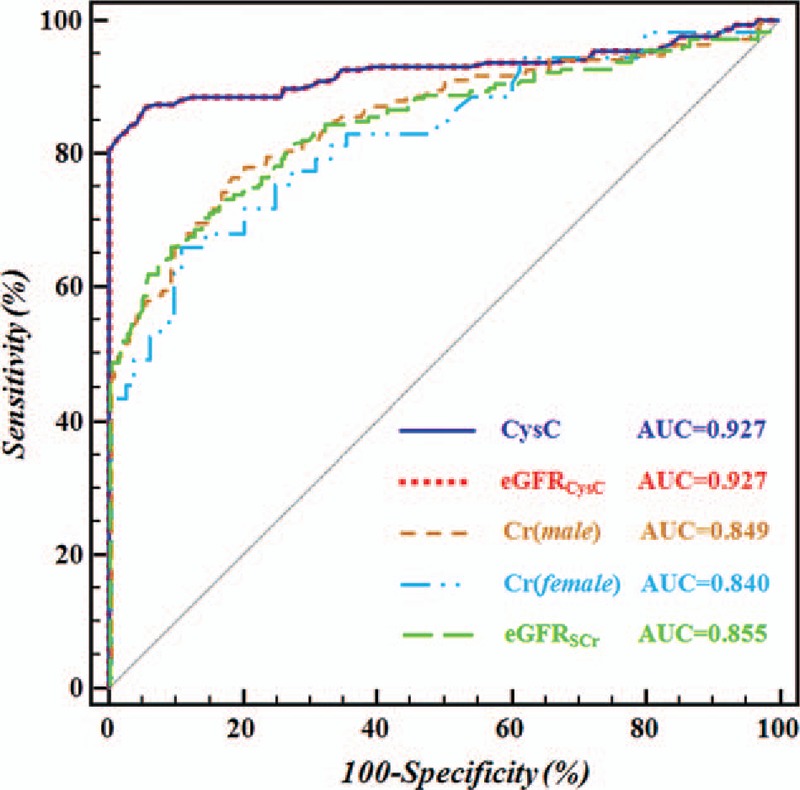
ROC curve for the evaluation of diagnostic efficiencies of CysC, eGFRCysC, Cr (man/woman) and eGFRSCr for kidney impairment in patients with hepatic cirrhosis. The diagnostic accuracy of each parameter, in terms of sensitivity and specificity, was presented after ROC curve analysis. The areas under the ROC curve (AUC) for CysC, eGFRCysC, Cr (man/woman) and eGFRSCr in the diagnosis of kidney injure was shown in hepatic cirrhosis patients. CysC = Cystatin C, eGFRcysc = eGFR was calculated on the basis of serum CysC, ROC = receiver operating characteristic.
Table 2.
Diagnostic efficiencies of CysC, eGFRCysC, SCr, and eGFRSCr for kidney impairment in patients with hepatic cirrhosis.
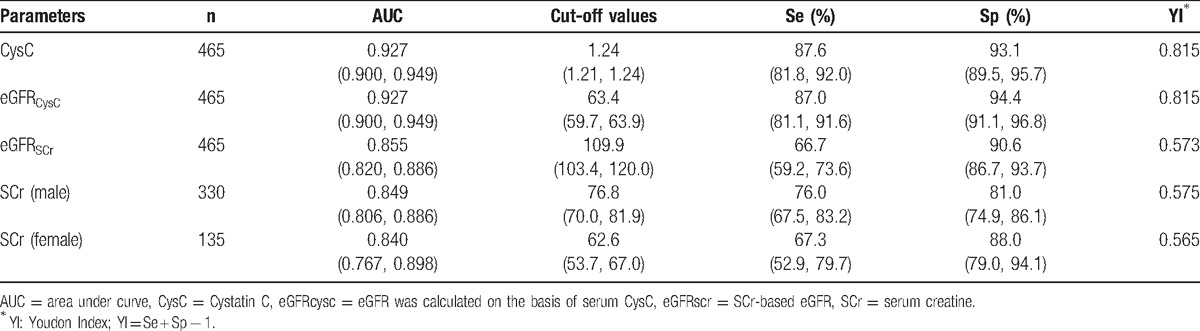
Table 3.
AUC of CysC, SCr, and eGFR in the diagnosis of kidney impairment.
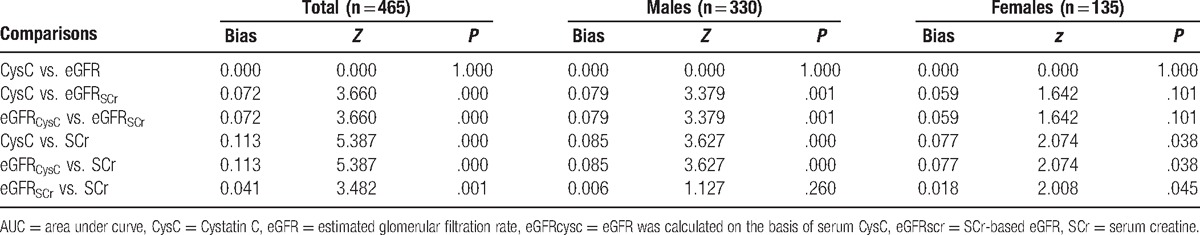
The diagnostic accuracy of each parameter, in terms of sensitivity and specificity, was presented after ROC curve analysis. The AUC for CysC, eGFRCysC, Cr(man/woman), and eGFRSCr in the diagnosis of kidney injure was shown in hepatic cirrhosis patients.
3.4. Diagnostic performances of SCr, CysC, and eGFR at different cut-off values
The upper reference limit (URL) was 1.09 mg/L for CysC and 97/71 μmol/L (man/woman) for SCr in our laboratory. Thus, the URL and above cut-off values were used as thresholds for the evaluation of diagnostic efficiencies of above parameters in kidney impairment of hepatic cirrhosis patients (Table 4).
Table 4.
Diagnostic performance of SCr, CysC, and eGFR at cut-off points.
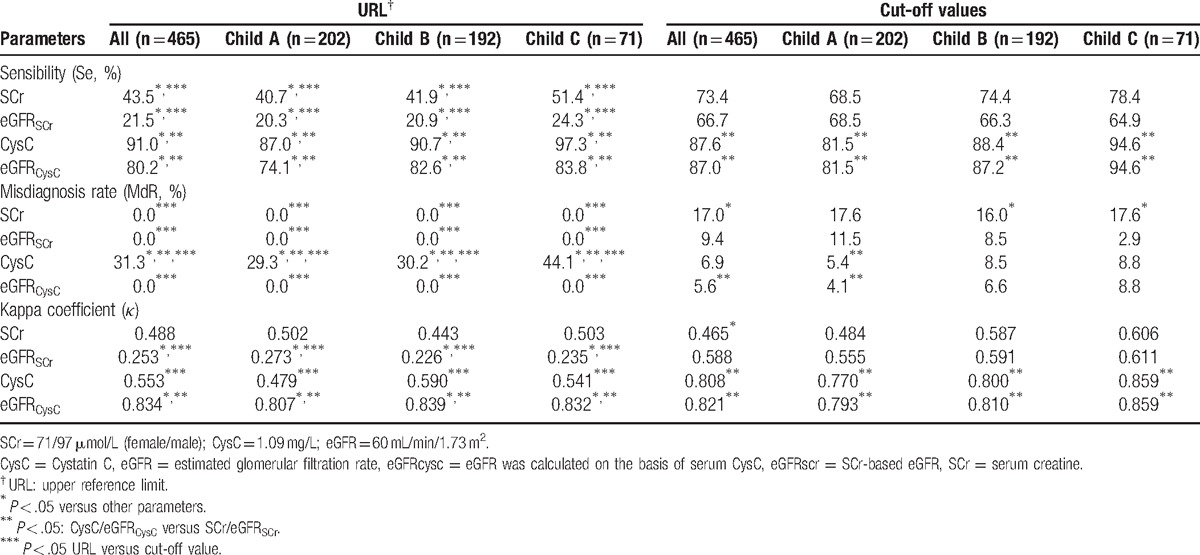
When the URL was used, CysC had the highest sensitivity (87.0%–97.3%) as well as the highest rate of misdiagnosis (29.3%–44.1%) in total patients and in patients of different Child-Pugh classes. The sensitivity and misdiagnosis rate of CysC were significantly different from those of eGFRCysC, SCr, and eGFRSCr (P < .05). Kappa test showed eGFRCysC had the best consistency between tentative diagnosis and clinical diagnosis of CKD (0.807–0.839), which was significantly higher than in CysC, SCr, and eGFRSCr (P < .05). The consistency coefficient of CysC, SCr, and eGFRSCr was 0.479 to 0.590, 0.443 to 0.503, and 0.226 to 0.273, respectively. The consistency coefficient of eGFRSCr was significantly lower than that of CysC and SCr (P < .05), but that was comparable between CysC and SCr (P > .05) (Table 4).
When the cut-off values of our study were used (the diagnostic potency was the best when the Youdon Index [YI] was the largest), the sensitivity, misdiagnosis rate, and kappa value were comparable between CysC and eGFRCysC (P > .05), the misdiagnosis rate was similar between SCr and eGFRSCr (P > .05), but the sensitivity and kappa value were markedly different between SCr and eGFRSCr (P < .05). When compared with CysC and eGFRCysC, SCr and eGFRSCr had significantly lower sensitivity and kappa value (P < .05) (Table 4).
In total patients and patients with different Child-Pugh classes, when the above cut-off values and upper limit were used in the diagnosis of CKD, the sensitivity and consistency (kappa test) of eGFRCysC were similar (P > .05); no significant difference was observed in the sensitivity of CysC and in the consistency (kappa test) of SCr (P > .05); significant difference was observed in the sensitivity and consistency (kappa test) of eGFRSCr (P < .05); there was significant difference in the misdiagnosis rate of 4 parameters (P < .05).
4. Discussion
In hepatic cirrhosis patients, the immune dysfunction and/or abnormal hemodynamics may cause kidney impairment via different ways,[14] or even result in HRS.[15,16] Thus, accurate evaluate of kidney impairment in hepatic cirrhosis patients is crucial for the maintenance of favorable kidney function and delaying of disease progression.[17] In the guideline on HRS of international organization of liver diseases[9] and guidelines on AKI and CKD of KDIGO, SCr is recommended for the laboratory diagnosis of kidney function. Thus, clinicians usually use SCr to reflect the kidney function in hepatic cirrhosis patients. However, our results showed the optimal cut-off value of SCr was 76.8/62.6 μmol/L (M/F) (Table 2) in the diagnosis of kidney impairment of patients with hepatic cirrhosis, which was significantly lower than the upper reference limit (97.0/71.0 μmol/L [M/F]) determined in our lab. It is suggested that kidney impairment precedes the change in SCr in hepatic cirrhosis patients and SCr fails to diagnose the kidney impairment at early stage in these patients. At this optimal cut-off value, the diagnostic sensitivity, misdiagnosis rate, and diagnostic consistency of SCr/eGFRSCr were consistently lower than those of CysC/eGFRCysC (P < .05) in total patients and those with different Child-Pugh classes, indicating that SCr/eGFRSCr is inferior to CysC/eGFRCysC in the diagnosis of kidney impairment. This may be also related to the difference in methodology besides the stability of creatinine influenced by multiple factors. Thus, in clinical practice, clinicians should concern the influence of methodology on the results in the evaluation of kidney function with SCr. Currently, enzyme method is used in the detection of SCr aiming to avoid the contamination by yellow picric acid and its orange products in the Jaff's method.[18–20] However, in a majority of available guidelines, SCr is detected with Jaff's method. Thus, the difference in methodology may bias the results.
Currently, nephrologists and clinical laboratory experts are being engaged in the exploration of the relationship between serum CysC and secondary kidney impairment in hepatic cirrhosis patients,[21–23] especially in those with normal SCr.[24] Available studies suggest that, as compared with SCr, serum CysC in a certain extent may be a more sensitive marker for assessment of kidney impairment in patients with liver cirrhosis. Our results showed the optimal cut-off value of CysC was 1.24 mg/L (corresponding to eGFRCysC at 63.9 mL/min/1.73 m2) (Table 2) in the diagnosis of kidney impairment in hepatic cirrhosis patients, which was higher than the URL (1.09 mg/L, corresponding to eGFRCysC at 72.4 mL/min/1.73 m2). Thus, if the URL was used, the results were over-estimated, resulting in increase in misdiagnosis rate. This may be ascribed to the difference in eGFR and actual GFR although there is no marked difference between them.[25,26] This study also implies that clinicians may not completely rely on the KDIGO guideline for the diagnosis and classification of severity of kidney disease with eGFR, and clinicians should determine the thresholds for the specific method used in their own laboratory.
KDIGO (2012) recommend the report of both eGFR and SCr. However, the eGFRSCr is dependent on the age and sex. Moreover, the method used to detect SCr changes (enzyme method is used in a majority of labs). Thus, the eGFRSCr is not reported in a variety of clinical labs (as least in China), and clinicians are accustomed to using the upper limit of SCr as a threshold in the diagnosis of kidney impairment. In the present study, results showed, when the upper limit of SCr was used, the sensitivity was as low as 40.7% to 51.4% in the diagnosis of patients with different Child-Pugh classes, which was significantly lower than that at the optimal cut-off value (P < .05) (Table 4). KDIGO guideline also suggests measuring CysC in adults when eGFRSCr is 45∼59 mL/min/1.73 m2 suggesting that the SCr alone may cause missed diagnosis in the diagnosis of secondary kidney impairment in hepatic cirrhosis patients. After adjustment for age and sex with c-aGFR, the calculated eGFRSCr may further reduce the diagnostic perform if the recommended cut-off value by KIDGO is sued (60 mL/min/1.73 m2), but its diagnostic performance may increase to that of CysC and eGFRCysC if the optimal cut-off value is used (109.9 mL/min/1.73 m2) (Table 2). However, in any laboratory, clinicians may not increase the cut-off value to 110 mL/min/1.73 m2 without any reason. Thus, if SCr or eGFRSCr is used for the diagnosis of kidney impairment, the likelihood of misdiagnosis is very high. In the laboratory evaluation of kidney function, SCr or eGFRSCr along with CysC should be measured together to ensure the accurate diagnosis.
Currently, it is the “gold standard” for GFR determination to measure the clearance of exogenous substances, such as inulin, iohexol, 99mTc-DTPA, 51Cr-EDTA, and 125I-iothalamate.[27] However, the wide application of these detections is significantly limited due to the risk for trauma, complex procedures, and/or radioactive contamination concern. Thus, eGFR has been recommended by KIDGO to replace GFR in the routine evaluation of renal filtration function. SCr has unavoidable disadvantages, and thus this study was undertaken to investigate the diagnostic efficiency of serum CysC, SCr, and their eGFR in kidney impairment of hepatic cirrhosis patients. Our results showed serum CysC and eGFRCysC were superior to SCr and eGFRSCr in the diagnostic performance of kidney impairment in these patients. A recent study also reveals that serum CysC based eGFR may more accurately reflect the GFR in hepatic cirrhosis patients[28] and thus can be used to evaluate the kidney function[29,30] and prognosis.[31] In addition, our findings also showed that eGFRCysC had the highest performance in the diagnosis of secondary kidney impairment and its optimal cut-off value was 63.4 mL/min/1.73 m2 (Table 2). Although this cut-off value was different from that recommended by KIDIGO (60 mL/min/1.73 m2), the sensitivity, misdiagnosis rate, and diagnostic consistency were similar at 2 cut-off values (P > .05). eGFR is not a measured parameter, which is calculated according to the SCr and/or CysC as well as other parameters in patients, thus, the results may be unavoidably affected by other factors. Thus, we speculate that, in the diagnosis of kidney impairment or classification of CKD with eGFR, the specific threshold determined in the individual lab should be used, and it is helpful for the accurate evaluation of kidney function.
Although CysC and eGFRCysC are superior to SCr and eGFRSCr in the diagnosis of kidney impairment, there is still risk for misdiagnosis and/or missed diagnosis when hepatic cirrhosis patients with secondary kidney impairment show slight reduction in GFR (GFR > 60 mL/min/1.73 m2). If above upper limit is used for the diagnosis, the misdiagnosis rate of CysC is higher than that of eGFRCysC (29.3%–44.1% vs. 0%, P < .05) and the diagnostic consistency of CysC is lower than that of eGFRCysC (0.479–0.590 vs. 0.807–0.839, P < .05) although the sensitivity of CysC is higher than eGFRCysC (87.0%–97.3% vs. 74.1%–83.8%, P < .05) (Table 4). This explains why international kidney disease organization recommends eGFR in the evaluation of kidney function.[7]
Of note, the Chronic Kidney Disease and Epidemiology (2012) recommends an equation employing both CysC and SCr which is developed on the basis of findings from western countries, and not applicable in Chinese population. Our previous study showed addition of SCr to the eGFRCysC equation failed to improve the diagnostic perform for CKD.[12] Thus, on the basis of simplicity, only CysC was used in the equation. In addition, eGFRCysC is estimated according to CysC and theoretically the ROCs of both parameters are assumed to be overlapped (Fig. 1). However, this was not observed in 3 present studies, which may be related to the choice of the decimal point after CysC being replaced with eGFRCysC. In addition, SCr is influenced by sex, which is not found in eGFRSCr because the calculation of eGFR with c-aGFR equation undergoes adjustment for sex. Furthermore, this is not differentiated between men and women in the guideline on CKD. Thus, in this study, it was combined with eGFR.
In conclusion, serum CysC and eGFRCysC are superior to SCr and eGFRSCr in the diagnosis of secondary kidney impairment of hepatic cirrhosis patients. Moreover, eGFRCysC is superior to CysC. However, eGFRCysC is not a measured parameter, and thus we recommend the individual cut-off value determined in the specific lab. In the laboratory evaluation of kidney function, clinicians should emphasize eGFR, but not focus on the measured parameter alone. Of note, clinicians should know that the methods used for the parameters and the experimental errors may bias the results when the kidney function is evaluated with eGFR.
Footnotes
Abbreviations: AKI = acute kidney injury, AUC = area under curve, CKD = chronic kidney disease, CV = coefficient of variation, CysC = Cystatin C, eGFR = estimated glomerular filtration rate, eGFRcysc = eGFR was calculated on the basis of serum CysC, eGFRscr = SCr-based eGFR, GFR = glomerular filtration rate, HRS = hepatorenal syndrome, KDIGO = kidney disease improving global outcomes, OEM = original equipment manufacture, PT = prothrombin time, SCr = serum creatine, URL = upper reference limit.
DW and J-FF have contributed equally to the article.
The study was supported in part by the Health and Family Planning Commission of Sichuan Province (No. 020089 and 150175). The funding agency had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors report no conflicts of interest.
References
- [1].Li X, Wang XK, Chen B, et al. Computational hemodynamics of portal vein hypertension in hepatic cirrhosis patients. Biomed Mater Eng 2015;26(Suppl):S233–43. [DOI] [PubMed] [Google Scholar]
- [2].Rognant N, Lemoine S. Evaluation of renal function in patients with cirrhosis: where are we now? World J Gastroenterol 2014;20:2533–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Risor LM, Bendtsen F, Moller S. Immunologic, hemodynamic, and adrenal incompetence in cirrhosis: impact on renal dysfunction. Hepatol Int 2015;9:17–27. [DOI] [PubMed] [Google Scholar]
- [4].Moller S, Krag A, Bendtsen F. Kidney injury in cirrhosis: pathophysiological and therapeutic aspects of hepatorenal syndromes. Liver Int 2014;34:1153–63. [DOI] [PubMed] [Google Scholar]
- [5].Beben T, Rifkin DE. GFR estimating equations and liver disease. Adv Chronic Kidney Dis 2015;22:337–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Adebisi SA. Routine reporting of estimated glomerular filtration rate (eGFR) in African laboratories and the need for its increased utilisation in clinical practice. Niger Postgrad Med J 2013;20:57–62. [PubMed] [Google Scholar]
- [7].Larsson A, Flodin M, Hansson LO, et al. Patient selection has a strong impact on cystatin C and Modification of Diet in Renal Disease (MDRD) estimated glomerular filtration rate. Clin Biochem 2008;41:1355–61. [DOI] [PubMed] [Google Scholar]
- [8].Stevens LA, Zhang Y, Schmid CH. Evaluating the performance of equations for estimating glomerular filtration rate. J Nephrol 2008;21:797–807. [PMC free article] [PubMed] [Google Scholar]
- [9].Runyon BA. Management of adult patients with ascites due to cirrhosis: an update. Hepatology 2009;49:2087–107. [DOI] [PubMed] [Google Scholar]
- [10].Pugh RN, Murray-Lyon IM, Dawson JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973;60:646–9. [DOI] [PubMed] [Google Scholar]
- [11].Malinchoc M, Kamath PS, Gordon FD, et al. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology 2000;31:864–71. [DOI] [PubMed] [Google Scholar]
- [12].Feng JF, Qiu L, Zhang L, et al. Multicenter study of creatinine- and/or cystatin C-based equations for estimation of glomerular filtration rates in Chinese patients with chronic kidney disease. PLoS ONE 2013;8:e57240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ma YC, Zuo L, Chen JH, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol 2006;17:2937–44. [DOI] [PubMed] [Google Scholar]
- [14].Karvellas CJ, Durand F, Nadim MK. Acute kidney injury in cirrhosis. Crit Care Clin 2015;31:737–50. [DOI] [PubMed] [Google Scholar]
- [15].Allegretti AS, Ortiz G, Wenger J, et al. Prognosis of acute kidney injury and hepatorenal syndrome in patients with cirrhosis: A prospective cohort study. Int J Nephrol 2015;2015:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Afinogenova Y, Tapper EB. The efficacy and safety profile of albumin administration for patients with cirrhosis at high risk of hepatorenal syndrome is dose dependent. Gastroenterol Rep 2015;3:216–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Belcher JM. Acute kidney injury in liver disease: role of biomarkers. Adv Chronic Kidney Dis 2015;22:368–75. [DOI] [PubMed] [Google Scholar]
- [18].Drion I, Cobbaert C, Groenier KH, et al. Clinical evaluation of analytical variations in serum creatinine measurements: Why laboratories should abandon Jaffe techniques. BMC Nephrol 2012;13:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cheuiche AV, Soares AA, Camargo EG, et al. Comparison between IDMS-traceable Jaffe and enzymatic creatinine assays for estimation of glomerular filtration rate by the CKD-EPI equation in healthy and diabetic subjects. Clin Biochem 2013;46:1423–9. [DOI] [PubMed] [Google Scholar]
- [20].Hermida FJ, Lorenzo MJ, Perez A, et al. Comparison between ADVIA Chemistry systems Enzymatic Creatinine_2 method and ADVIA Chemistry systems Creatinine method (kinetic Jaffe method) for determining creatinine. Scand J Clin Lab Invest 2014;74:629–36. [DOI] [PubMed] [Google Scholar]
- [21].Barakat M, Khalil M. Serum cystatin C in advanced liver cirrhosis and different stages of the hepatorenal syndrome. Arab J Gastroenterol 2011;12:131–5. [DOI] [PubMed] [Google Scholar]
- [22].Adachi M, Tanaka A, Aiso M, et al. Benefit of cystatin C in evaluation of renal function and prediction of survival in patients with cirrhosis. Hepatol Res 2015;45:1299–306. [DOI] [PubMed] [Google Scholar]
- [23].Culafic D, Stulic M, Obrenovic R, et al. Role of cystatin C and renal resistive index in assessment of renal function in patients with liver cirrhosis. World J Gastroenterol 2014;20:6573–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kim DJ, Kang HS, Choi HS, et al. Serum cystatin C level is a useful marker for the evaluation of renal function in patients with cirrhotic ascites and normal serum creatinine levels. Korean J Hepatol 2011;17:130–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lamb EJ, Stevens PE. Estimating and measuring glomerular filtration rate: methods of measurement and markers for estimation. Curr Opin Nephrol Hypertens 2014;23:258–66. [DOI] [PubMed] [Google Scholar]
- [26].Lemoine S, Guebre-Egziabher F, Sens F, et al. Accuracy of GFR estimation in obese patients. Clin J Am Soc Nephrol 2014;9:720–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Florkowski CM, Chew-Harris JS. Methods of Estimating GFR—different equations including CKD-EPI. Clin Biochem 2011;32:75–9. [PMC free article] [PubMed] [Google Scholar]
- [28].Mindikoglu AL, Dowling TC, Weir MR, et al. Performance of chronic kidney disease epidemiology collaboration creatinine-cystatin C equation for estimating kidney function in cirrhosis. Hepatology 2014;59:1532–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Vukobrat-Bijedic Z, Husic-Selimovic A, Mehinovic L, et al. Estimated glomerular filtration rate (eGFR) values as predictor of renal insufficiency in advanced stages of liver diseases with different etiology. Med Arh 2014;68:159–62. [PubMed] [Google Scholar]
- [30].Uguen T, Jezequel C, Ropert M, et al. Pretransplant renal function according to CKD-EPI cystatin C equation is a prognostic factor of death after liver transplantation. Liver Int 2015;36:547–54. [DOI] [PubMed] [Google Scholar]
- [31].Cholongitas E, Arsos G, Goulis J, et al. Glomerular filtration rate is an independent factor of mortality in patients with decompensated cirrhosis. Hepatol Res 2014;44:E145–55. [DOI] [PubMed] [Google Scholar]


