FIG. 2.
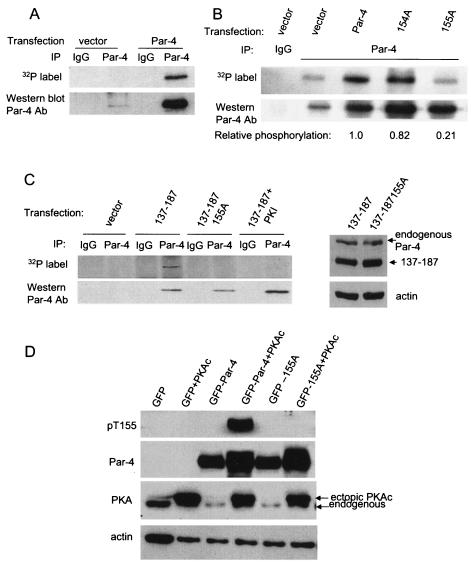
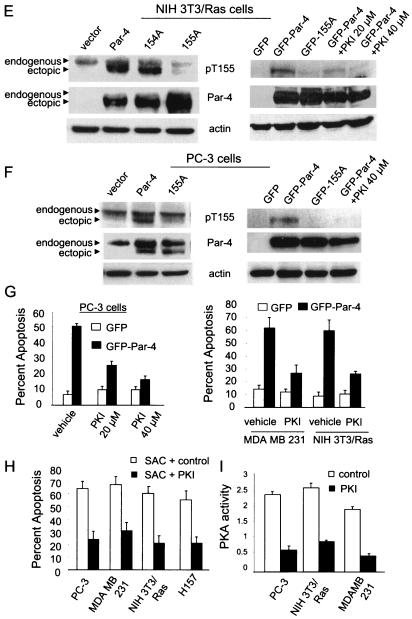
PKA phosphorylates Par-4 at T155. To determine if Par-4 is a phosphorylated protein (A), NIH 3T3/Ras cells were transiently trasfected with vector or Par-4 for 12 h, incubated in phosphate-free medium overnight, and then metabolically labeled with 200 μCi of [32P]orthophosphate for 5 h. Whole-cell extracts were subjected to immunoprecipitation with Par-4 or control IgG antibody, resolved by SDS-PAGE, and transferred to a PVDF membrane. The blot was autoradiographed (top) and finally probed with the Par-4 antibody (bottom). To determine whether PKA phosphorylates Par-4 at T155 (B), NIH 3T3 cells were transiently transfected with vector, Par-4, 154A, or 155A expression construct for 24 h, and whole-cell extracts were immunoprecipitated with the Par-4 antibody or IgG control antibody. The immunoprecipitated proteins were subjected to an in vitro phosphorylation reaction with purifed PKA enzyme and [γ-32P]ATP, and then the radiolabeled proteins were resolved by SDS-PAGE, transferred to a PVDF membrane, and autoradiographed. The blot was finally probed with Par-4 antibody to determine Par-4 or mutant protein levels. Following densitometric scanning of the blots, the amount of 32P label incorporated into ectopic Par-4 or mutant protein by PKA was normalized to the corresponding protein level detected on the Western blots, and the relative amount of 32P label incorporated (i.e., PKA phosphorylation) is shown at the bottom of the panel (B). In experiments aimed at determining whether PKA phosphorylates Par-4 in vivo (C), we used 137-187 and 137-187/155A expression constructs, which were first tested for expression by transient transfection, followed by Western blot analysis with Par-4 or actin antibody (C, right). To examine whether PKA phosphorylates Par-4 in vivo (C, left), NIH 3T3/Ras cells were transiently transfected with a vector or 137-187 or 137-187/155A mutant expression constructs in the presence or absence of PKA-inhibitory peptide PKI for 24 h, then metabolically labeled, and immunoprecipitated with the Par-4 antibody or IgG control antibody. The immunoprecipitates were subjected to SDS-PAGE, transferred to nylon membranes, and autoradiographed (C, top left). Finally, the blots were probed with the Par-4 antibody (C, bottom left), as described above (A and B). To characterize the phospho-T155 (pT155) antibody, MEFs were transfected with GFP, GFP-Par-4, or GFP-155A plasmids with or without the PKAc expression construct for 24 h, and Western blot analysis for phospho-T155, Par-4, the PKA catalytic subunit, and actin was performed (D). NIH 3T3/Ras (E) or PC-3 (F) cells were transiently transfected with vector, Par-4, 154A, or 155A plasmids (E and F, left), or with GFP, GFP-Par-4, or GFP-Par-4/155A plasmid; the cells were then treated for 24 h with vehicle or 20 or 40 μM PKI (E and F, right) and then subjected to Western blot analysis with pT155, Par-4, or actin antibodies. To determine whether inhibition of PKA activity inhibits apoptosis by Par-4 (G), PC-3, NIH 3T3/Ras, or MDA MB 231 cells were transiently transfected with GFP or GFP-Par-4. The PC-3 transfectants were treated with vehicle or with 20 or 40 μM PKI; NIH 3T3/Ras or MDA MB 231 cells were treated with 20 μM PKI. The transfectants and cells were then assayed for apoptosis, as described in the legend to Fig. 1. To ascertain that inhibition of PKA activity by PKI inhibits apoptosis by the SAC domain in diverse cancer cell lines, the cells were transfected with the SAC expression construct, treated with 20 μM PKI peptide or control, and scored for apoptosis (H). To confirm that PKI inhibits PKA activity, PC-3, NIH 3T3/Ras, or MDA MB 231 cells were treated with control or 20 μM PKI peptide for 48 h, and whole-cell lysates were tested for PKA activity with the cAMP-dependent PKA Signatect assay kit (I).