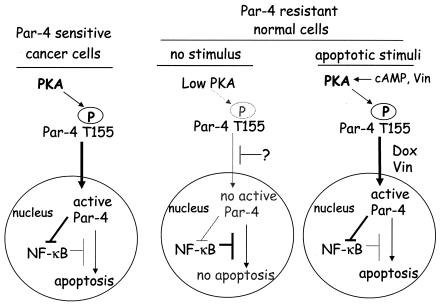
FIG. 6.
Schematic representation of PKA- and T155-dependent apoptosis by Par-4 in normal and cancer cells. Based on our findings, we propose the following model for differential apoptosis by Par-4 in cancer and normal cells. PKA activity, which is constitutively elevated in cancer cells, causes phosphorylation of Par-4 at the T155 residue. The translocation of phospho-T155 to the nucleus results in inhibition of NF-κB activity and apoptosis. By contrast, PKA activity and T155 phosphorylation levels are relatively low in normal cells. In addition, Par-4 translocation to the nucleus is blocked by an unknown mechanism. Consequently, normal cells fail to undergo apoptosis with Par-4. However, proapoptotic stimuli, such as cAMP or vincristine, cause PKA-dependent phosphorylation of T155; doxorubicin or vincristine can cause translocation of Par-4 to the nucleus. Therefore, vincristine alone or a combination of cAMP and doxorubicin can induce apoptosis of the normal cells in a PKA- and T155 Par-4-dependent mechanism. The mechanism of nuclear translocation of Par-4 is currently under investigation.