Abstract
Context
Bladder cancer therapy remains suboptimal as the morbidity and mortality remains high amongst those with non-muscle invasive and muscle-invasive disease. Regional hyperthermia therapy (RHT) is a promising adjunctive therapy being tested in multiple clinical contexts.
Objective
To systematically review the literature on the efficacy and toxicity of RHT.
Evidence Acquisition
The systematic review was registered with the PROSPERO database (Registration number: CRD42015025780) and was conducted according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines. We queried PubMed, EMBASE, and Cochrane libraries. Two reviewers reviewed abstracts independently and a third reviewer arbitrated disagreements. The last search was performed on August 28, 2015. A descriptive analysis was performed and quality assessment was conducted using the Newcastle-Ottawa Quality Assessment Scale for observational studies, and the Cochrane Risk of Bias Assessment Tool for trials.
Evidence Synthesis
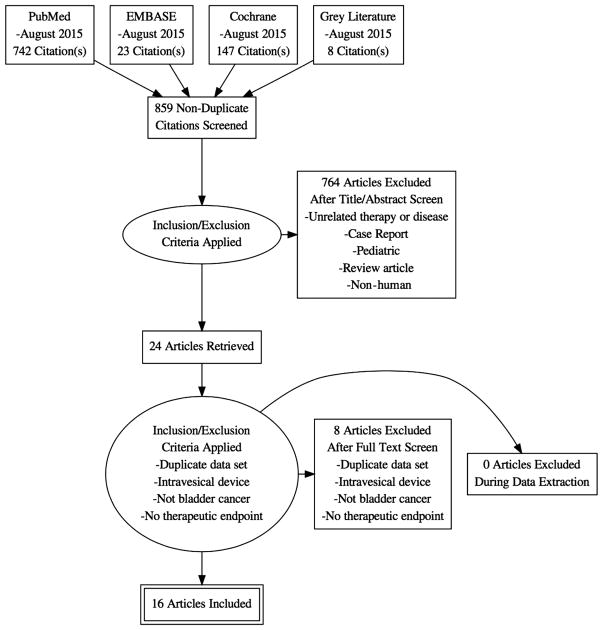
We identified 859 publications in the initial search, of which 24 met inclusion criteria for full-text review. Of these, we were able to obtain data on the outcomes of interest for 15 publications.
Conclusions
The review underscores the limited nature of the evidence; definitive conclusions are elusive. However, the promising results of RHT in the setting of intravesical chemotherapy, chemotherapy and radiotherapy show a trend towards legitimate efficacy.
Keywords: Bladder cancer, urothelial carcinoma, regional hyperthermia therapy, radiohyperthermia, chemohyperthermia
1. Introduction
There will be an estimated 74,000 new cases of bladder cancer and 16,000 deaths in the United States in 2015. This represents 4.5% of all new cancers in the US and it remains the 5th most common [1]. Over 75% are non-muscle invasive bladder cancer (NMIBC) and of these, 50% will recur after the transurethral resection of bladder tumor (TURBT) [2]. Caring for NMIBC involves continued surveillance and frequently, intravesical therapy. Intravesical therapy with chemotherapy and/or immunotherapy has suboptimal efficacy and some toxicity. Especially the patients that are at high risk for a recurrence are found to be refractory to this form of therapy and often proceed to radical cystectomy [3]. Muscle invasive bladder cancer (MIBC) often leads to a cystectomy per guidelines and the associated significant morbidity and mortality [4, 5]. Patients are faced with the anxiety and physical burden, not to mention that bladder cancer is the most expensive malignancy per patient [6]. Novel therapies are being sought as ways to reduce the morbidities and costs of the disease, and potentially preserve that patient’s native bladder.
Pre-clinical data provide strong evidence that hyperthermia has the potential to induce anti-tumour immunity [7]. There are at least five proposed mechanisms for the activation of the immune response by hyperthermia. 1) Tumor cells increase surface expression of several markers (e.g., MHC class I) when exposed to heat. 2) Heat causes the tumor to release HSPs, which in turn activates the host immune response. 3) Heated tumor cells release exosomes that carry tumor antigens to the immune system. 4) Heat alone directly activates the immune system. 5) Heat renders the tumor vasculature more permeable which allows for better trafficking of immune cells [8].
Additionally, hyperthermia is an interesting topic of research because of the sensitizing effect with chemotherapy and/ or radiotherapy. Hyperthermia has been shown to enhance drug delivery and thermosensitizes cancer cells to certain antineoplastic drugs [9, 10]. In bladder cancer, it leads to chemosensitization by impairing the repair of damaged DNA and has synergistic action with cytotoxic agents, such as Mitomycin C [11, 12].
Broadly, hyperthermia of the bladder can be achieved via different methods: radiofrequency emitting intravesical catheters (e.g. Synergo, operating at a frequency of 915 MHz [10, 13, 14], externally heated chemotherapy fluid circulation in the bladder [15], intravesical magnetic nanoparticles [16], and external deep regional radiofrequency transmission with 70–110 MHz [17]. While the intravesical heating methods are more commonly used and produce uniform temperatures across the urothelial lining of the bladder, there is a significant temperature gradient measured through increasing depth of the bladder wall. This temperature gradient is unlikely to be important for the treatment of NMIBC (which is confined to the urothelium and suburothelial lamina propria), but may have important consequences for the treatment of muscle invasive bladder tumors. Clinical evidence for the positive effect of radiofrequency-induced hyperthermia (operating at 915 MHz) was published in a meta-analysis in Ta-1 G1-3 NMIBC. After the combination of heat and intravesical therapy with Mitomycin C (MMC), a 59% relative reduction in NMIBC recurrence was seen compared to MMC alone [18]. According to progression, lower rates were seen in case of chemohyperthermia although in most studies an adequate follow up was lacking [19]. Regional hyperthermia therapy (RHT) results in a more uniform temperature distribution across the bladder wall and the field can be directed beyond the bladder itself to the lymphatic drainage. There are severally commercially available RHT devices including the AMC device, BSD devices, and Thermotron device.
The BSD-2000 (BSD Medical, Salt Lake City, UT, USA) (and its predecessor, the BSD-1000) has been one of the most widely studied devices for RHT. It has been used in trials alone, and as an adjunct to surgery, radiation, and chemotherapy [17,20–23]. This system delivers RHT emitting electromagnetic radiofrequency radiation using a 100±2 MHz radiofrequency Sigma Eye twenty-four array of dipole antennas that surround the patient as the applicator. Applicator selection is based on patient size. Proprietary software pre-plans treatment sessions. The bladder temperature can then be measured through the Foley catheter to assure that the target temperature of 40–45°C is achieved [24]. The patient is then treated for a duration of 45–60 minutes once the therapeutic temperature is met. System power, frequency, relative antenna power, and phase were adjusted frequently during treatment to optimize bladder heating and patient tolerance based on measured temperatures, blood pressure, and patient verbal feedback.
The AMC 70 MHz phased array system consists of one or two rings, each with 4 wave guide antennas (aperture 33 × 21 cm) positioned around the pelvis of the patient and connected to a phase and amplitude controlled RF Generator (SSB Electronic). Patients are treated in supine position, using cooled water pillows to connect the antenna power to the patient. Phase settings are first optimized to create a focus at the rectum guided by an E-field probe in the rectum, followed by a phase shift of the dorsal and ventral antenna to move the focus from rectum to bladder [25].
Final settings are validated by performing ΔT pulses for three phase settings with 40° phase shifts between the dorsal and ventral antenna [26].
Thermometry is performed with 14-sensor thermocouple probes (Ella CS, Czech Republic) inserted in catheters in bladder, rectum (and vagina, if applicable). Temperature data are recorded using a 196-channel thermometry unit. Treatment continued for 60 min after bladder temperatures reached 41°C, or for a maximum total duration of 90 min, whichever was shortest [17, 27, 28].
The Thermotron (Yamamoto Vinita, Co., Osaka, Japan) is an 8 MHz radiofrequency capacitive heading device. The output ranges from 271–965 (578 ±86.2) Watt. Large, deep seated tumors require large electrodes, and conversely small, superficial tumors respond to smaller electrodes. Pairs of electrodes with a cooling bolus are placed on the front and back of the patient. Patients with subcutaneous fat tissue > 2 cm thickness had superficial tissue was cooled for 15–20 min prior to and during heating with 10–15°C saline. For heating deep areas of the body, an overlay bolus sheet in addition to the regular bolus was applied and the body surface was cooled continuously during heating [29, 30].
This collaborative review provides a critical overview of current literature concerning the role of deep regional hyperthermia for the treatment of NMIBC and MIBC.
2. Evidence Acquisition
The systematic review was registered with the PROSPERO database (Registration number: CRD42015025780) and was conducted according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines. A search of Medline, Embase, Cochrane Library, CancerLit and ClinicalTrials.gov databases was undertaken for candidate manuscripts from 1966 through August 2015 and was not limited by language.
Candidate manuscripts were reviewed according to the Cochrane Collaboration criteria and reported following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines.
Two reviewers (TL and AG) independently performed database searches, assessed candidate manuscripts for inclusion criteria, and extracted primary data. A detailed description of the search strategy is given in Appendix A. Authors and experts in the field were asked for additional studies. In order to address a paucity of data on this topic, this systematic review followed the best-available-evidence approach and included several single group studies, a decision made a priori [31]. Reviewed studies consist of prospective and retrospective cohorts in which all subjects received a single intervention and the outcome was assessed over time, but lacks an internal comparison.
2.1 Eligibility criteria
Studies were eligible if they met the following inclusion criteria: 1) Adult patients with diagnosis of bladder cancer, 2) intervention with regional hyperthermia.
Studies were excluded if they involved: 1) pediatric patients, 2) animal studies, or 3) review papers 4) case reports, or 5) ablative temperatures > 45C. The review was not restricted to the English language. Duplicate publications were also excluded and only manuscripts where the full-text publication was retrievable were included.
2.2 Quality assessment
Study quality assessment was conducted using the Newcastle-Ottawa Quality Assessment Scale, which is used to evaluate studies based on three criteria: (i) patient selection, (ii) comparability of groups, and (iii) ascertainment of outcome or the Cochrane Risk of Bias Tool for clinical trials. Studies were assessed on a star scoring scale, with higher scores given for higher quality studies. Studies were not excluded in the review based on the perceived quality of the study. The primary end point was response. The secondary end points were recurrence, time to progression and adverse event (AE) rate.
3. Evidence synthesis
3.1 Search results
We identified 859 publications in the initial search, of which 24 met inclusion criteria for full-text review. Of these, we were able to obtain data on the outcomes of interest for 15 publications. A total of 346 patients who underwent RHT treatment for bladder cancer were included. Characteristics of the studies which were included can be found in Table 1.
Table 1.
Overview of all trials concerning regional hyperthermia therapy for bladder cancer
| Reference | Study Type (retro vs. pro) | Number of patients | Patient Population (NMIBC, MIBC) | Study Description | Type | Device | Temperature °C | CR | PR |
|---|---|---|---|---|---|---|---|---|---|
| Geijsen et al.[26] | Prospective | 18 | NMIBC | Pilot: Phase I/II | HT plus intravesical MMC | AMC | 40.6–42.1 | ||
| Hisazumi et al.[33] | Retrospective | 39 | MIBC, NMIBC | Case Series | Eight-MHZ RF | 9 (23%) | 6 (15%) | ||
| 13 NMIBC | HT | Thermotron | 0 | 0 | |||||
| Radiohyperthermia | 2 (15%) | 0 | |||||||
| Chemohyperthermia | 4 (31%) | 0 | |||||||
| 26 MIBC | HT | Thermotron | 0 | 0 | |||||
| Radiohyperthermia | 3 (12%) | 5 (19%) | |||||||
| Chemohyperthermia | 0 | 1 (4%) | |||||||
| Inman et al.[20] | Prospective | 15 | NMIBC | pilot | HT plus intravesical MMC | BSD 2000 | Goal 42±2 | ||
| Masunaga et al.[36] | Prospective | 28 | MIBC, NMIBC | Phase I and II Trial | Radiohyperthermia vs radiation | Thermotron | 39.4–44.0 | ||
| Naito et al.[35] | Retrospective | 21 | MIBC | Case Series | Chemohyperthermia, radiohyperthermia | 0 | 10 (48%) | ||
| Nishimura et al.[38] | Retrospective | 4 | MIBC | Case Series | Radiohyperthermia | Thermotron | Average 38–43 | 2 (50%) | 1 (25%) |
| Noguchi et al.[32] | Prospective | 35 | MIBC, NMIBC | Pilot | Quadrimodal vs radiohyperthermia | Radiofrequency apparatus | Goal 42 | 8 (23%) | 12 (34%) |
| 18 MIBC, NMIBC | Radiohyperthermia | 4 (22%) | 7 (39%) | ||||||
| 17 MIBC, NMIBC | Chemoradiohyperthermia | 4 (23%) | 5 (29%) | ||||||
| Ohguri et al.[39] | Retrospective | 3 | MIBC | Case Series | Chemoradiohyperthermia | Thermotron | Average/patient 43.7–45.5 | 3 (100%) | 0 |
| Petrovich et al.[21] | Prospective | 20 | Phase I | Hyperthermia plus radiation | BSD 1000 | Goal 42.5 | 0 | 2 (10%) | |
| Rietbroek et al.[27] | Prospective | 4 | MIBC | Pilot | Chemohyperthermia | AMC | Goal 41.0 | 0 | 2 (50%) |
| Sapozink et al.[22] | Prospective | 5 | MIBC | Pilot | Radiohyperthermia | BSD 1000 | Goal ≥42.0 | 2 (40%) | 0 |
| Uchibayashi et al.[37] | Prospective | 46 | MIBC | cohort | Chemohyperthermia or radiohyperthermia | Radiofrequency apparatus | Goal 42.5 | 5 (11%) | 15 (33%) |
| 27 MIBC | Chemohyperthermia | 3 (11%) | 8 (30%) | ||||||
| 19 MIBC | Radiohyperthermia | 2 (11%) | 7 (37%) | ||||||
| Ueda et al.[31] | Prospective | 11 | NMIBC | cohort | HT plus intravesical doxorubicin | Thermotron | Goal ≥42.0 | 6 (54%) | 3 (27%) |
| Van der Zee et al.[18] | Prospective | 52 | MIBC | Randomized | Radiohyperthermia vs radiation | BSD, and others | Goal ≥42.0 | 38 (73%) | |
| Wittlinger et al.[19] | Prospective | 45 | MIBC, NMIBC | Phase II | Quadrimodal | BSD 2000 | Goal 41.5 | 36 (80%) | 45 (96% |
3.2 Non-muscle invasive bladder cancer
There were two pilot studies using RHT with intravesical mitomycin C (MMC) in NMIBC [21, 27], and a third trial using intravesical doxorubicin [32]. Geijsen et al. employed the AMC 70 MHz device and involved 6 weekly courses of RHT combined with intravesical chemotherapy with MMC in 18 patients with intermediate and high risk NMIBC after trans urethral resection of the bladder tumor; it was then followed with a maintenance period over the next 12 months. During maintenance, the patients received a single course of RHT with MMC at 3, 6, 9, and 12 months [27]. The primary endpoint of this trial was feasibility and toxicity. No grade 3 toxicity or higher was seen and the temperatures achieved in the bladder were satisfactory. They reported a recurrence free survival rate of 78% at 24 months. However, 3/9 patients who had completed induction showed recurrent or progressive disease and one developed distant metastasis. 83% of the patients completed induction and 50% completed maintenance, or a 17% and 50% dropout rate, respectively.
Inman et al. assessed 15 NMIBC, in patients who were BCG refractory. The treatment protocol consisted of 6 weekly courses of RHT combined with intravesical MMC. A maintenance schedule involved monthly RHT with intravesical MMC for 4 additional months. 73% of patients completed induction and maintenance for a dropout rate of 27%. Recurrence-free survival at 24 months was 33%, but this was maintained beyond 3 years. None of the recurrences progressed to muscle invasive disease. Six of the ten patients who experienced recurrence underwent a cystectomy and all were node negative with no greater than T1 disease [21]. The difference in length of the treatment period may have influenced the lower dropout rate and lower recurrence free survival compared to Geijsen et al.
Ueda et al. performed a nonrandomized trial comparing intravesical HPC-doxorubicin in 20 patients to intravesical HPC-doxorubicin combined with RHT using the Thermotron device in 11 patients with NMIBC. A complete response was noted in 6/11 (54.5%) patients with combination therapy versus 7/20 (35%) in intravesical therapy alone. A partial response was seen in 27.3% versus 30% in combination versus intravesical alone [32]. The manuscript did not include results analyzed by grade, a mean follow up, or a recurrence rate.
Additionally, there were three studies that looked at both NIMBC and MIBC [20, 33, 34]. The efficacy and toxicity of these trials will be discussed below in the MIBC section.
Toxicity
Geijsen et al. determined toxicity would be a primary endpoint and used CTC 3.0 [35]. Over 10% of patients experienced a grade 2 toxicity comprised of bladder complaints and lower back pain. No grade 3 or 4 toxicities were seen, but 6/18 patients discontinued therapy because of physical complaints, including MMC allergy. No damage was observed cystoscopically. The secondary objective was bladder cancer recurrence and progression. Similarly, Inman et al. did not report any grade 3 or higher toxicities. The most common adverse event was grade 1 urethral discomfort (40%) followed by abdominal discomfort from the device (33%) [21]. Ueda et al. reported adverse events including dysuria and frequency. The intravesical alone group reported adverse effect rate of 25% and the cohort with RHT reported 27% [32].
In conclusion: In NMIBC only limited phase I/II studies have been performed using regional hyperthermia in combination with different schedules of intravesical chemotherapy and different therapy regimens making a real comparison difficult.
3.3 Muscle invasive bladder cancer
RHT has been used to treat MIBC alone and in combination with chemotherapy and radiation therapy. Early reports examined heterogeneous treatment protocols and vaguely defined categories of bladder cancer [34, 36]. More dedicated trials examined each individual combination.
3.4 Radiohyperthermia (RH)
There has only been one prospective, randomized controlled trial of RHT in MIBC as an adjunct to radiation therapy. Van der Zee et al. enrolled 358 patients with pelvic tumors of which 101 had MIBC. The patients were then randomized to radiotherapy alone (n= 49) or radiotherapy with hyperthermia (n=52). A CR was noted in 25/49 (51%) vs 38/52 (73%) in the radiotherapy versus combination groups respectively. The overall survival was 22% for radiotherapy and 28% for combination therapy at 3 year follow up; this difference was not significant [17].
There have been several observational cohorts examining a similar protocol of RH for a total of 63 patients. Masunaga et al performed a clinical trial comparing radiotherapy in 21 patients to RH in 28 patients (6 NMIBC 22 MIBC) using the Thermotron device prior to planned cystectomy [37]. All patients received 24 Gy of radiation (4 Gy/day on 3 days/week) either alone, or in combination with hyperthermia. They then underwent a surgical resection within a week of completed this preoperative therapy. There were no local recurrences in the group treated with hyperthermia, while there was one instance in the radiotherapy alone group. Both groups experienced distant metastasis without a significant difference in numbers (2 in radiotherapy alone, 4 in RH). Although the survival rate was higher in the RH group, they were unable to show statistical significance, probably due to the small number of patients. In another study in Japan, Uchibayashi et al. performed a trial with a cohort involving RH (mean 49±3 Gy) in 19 patients. There was a CR in 3/19 patients and a PR of 8/19 patients. The survival period was 65, 31.5 and 11.6 months for T2, T3, T4 disease respectively [38]. Sapozink et al. performed a pilot study with the BSD device on 5 bladder cancers [23], and Nishimura et al. had a retrospective case series that applied RHT and radiation (40–50 Gy) to 4 bladder tumors [39]. There were 5 complete responses among these patients without mention of the tumor stage. Noguchi et al. compared a cohort of patients with RH to another cohort of RH (40 Gy) plus chemotherapy (RH+MVAC). The response rates between the groups were similar; a CR of 4/17 and 4/18 and a PR of 5/17 and 7/18 for RH and RH+MVAC respectively [33]. Petrovich et al. examined 20 patients with urothelial cell that are described as advanced tumors. All patients received hyperthermia and this may have been alone or with chemotherapy or with radiotherapy. A complete response was not seen in any of the patients and only 2 partial responses were noted. Both of the PR had received radiation in addition to hyperthermia [22].
Toxicity
In Noguchi et al. both the RH and RH+MVAC groups two-thirds experienced tenesmus and frequency. Fewer than 20% experienced fatty necrosis [33]. They did report one case of a contracted bladder thought to be related to the hyperthermia but did not distinguish side effects by tumor type. Sapozink et al. did not distinguish amongst histology, but did note that the most common toxicities were pain and bladder spasm [23]. The RCT listed adverse events without a grade or distinction to tumor, but included subcutaneous burns in 20 patients, skin burns in 5 patients, and urinary tract infection [17].
3.5 Chemohyperthermia (CH)
There have been two publications looking specifically at RHT with intravenous chemotherapy. Rietbroek et al. employed the AMC 70 MHz device at weekly intervals with cisplatin for 4 patients with T3 or T4 disease for a minimum of 4 cycles. Two patients had partial response with a duration of 5 and 7 months [28]. Uchibayashi et al. performed a trial with two cohorts of MIBC; the CH cohort consisted of 19 patients receiving THP-adriamycin and 9 patients received platinum based chemotherapy. The other cohort involved RH and will be discussed below. There was a CR in 2/27 patients and a PR of 7/27 patients. The survival period in those treated with THP was 45.2, 15.4, and 13.5 months for T2, T3, T4 disease respectively, and for cisplatin 24, 36, and 8.5 months [38].
Toxicity
Rietbroek et al. evaluated toxicity using the WHO grading system, but did not report toxicities by tumor type. They did not report any grade 4 toxicities, and felt that the only hyperthermia related toxicities were fat necrosis (11%), 2 cases of skin burns, and most commonly, mild pain (31%) [28]. Uchibayashi et al. reported burns in 13/46 patients and anorexia in 11 patients [38]. Measured bladder temperatures were not given in relation to these toxicities.
3.6 Quadrimodal therapy
Several studies have looked at the combination termed quadrimodal therapy, defined as surgery (trans urethral resection of the bladder tumor, chemotherapy, radiation therapy, and RHT. Ohguri et al. used this therapy in order to preserve the bladders of three patients with MIBC (all T2 disease, one N0, 2 N1) [40]. This protocol involved higher temperatures (average from 44.5, 47, 47.4) than is typically achieved in the majority of trials, but was chosen for better local response. The hyperthermia was given immediately following radiotherapy (total dose 66–70 Gy) during the course of chemotherapy. All three patients achieved a complete response without any recorded recurrence or metastasis (16, 18, and 40 months).
As mentioned earlier, Wittlinger et al. examined quadrimodal therapy in MIBC. Their protocol was more defined than the cases described by Ohguri et al. and involved 19 patients with T2 MIBC in addition to 26 high risk NMIBC patients. All patients underwent a TURB. In the entire trial 15/45 patients completed less than the minimum number of RHT sessions [20]. At the initial re-TURBT, 96% of patients had a complete response and recurrence free survival was 81% at three years. There were 5 disease specific deaths, all of which involved distant metastases with complete local control. Disease specific survival was 88% at 3 years and overall survival as 80% at 3 years while disease free survival and metastasis free survival were 71% and 89% respectively. The 3 year bladder preservation rate was 97% for those alive, and 96% overall.
Toxicity
In Ohguri et al. no grade 3 or greater toxicity was reported, but all patients experienced cystitis [40]. Wittlinger et al. recorded toxicity using National Cancer Institute Common Terminology Criteria (CTCAE), version 3.0 [20, 35]. Grade 3 and 4 sequelae were reported in 24% of patients. Specifically, 1 salvage cystectomy for hematuria, 1 contracted bladder (<100), a reduced bladder capacity (100–200 mL) was seen in 13% of patients, and 2 bowel obstructions [20].
4. Limitations
This systematic review represents a comprehensive summary of a clinically relevant topic. The inclusion of single group studies suffers from several drawbacks. The lack of internal comparisons limits the role for inference of the comparative effectiveness, and alternative explanations should be considered for the effects seen other than the intervention being studied. However, single arm reviews are appropriate for the identification and possible quantification of harms. They are common in the evaluation of novel technology and compose the majority of available data. The inclusion of such studies is common amongst systematic reviews and criteria for inclusion have been proposed by Ip et al [41]. The choice of a proxy comparison is open to bias because it depends upon assumptions. For many of the studies without an internal control, the unlikelihood of spontaneous regression of disease coupled with the documented response rates is informative. Comparisons of adverse events are difficult because the older studies used non-validated questionnaires, whereas more recent studies used Common Toxicity Criteria for Adverse Events. There is also inconsistent analysis amongst the trials. The populations examined have heterogeneity in demographic factors (particularly race and functional status), and span a large period of time. Similarly, there was no standardized methodology.
5. Conclusions
Our systematic review suggests that with regional hyperthermia high bladder temperatures can be achieved with acceptable toxicity. It may be effective in both the NMIBC and MIBC setting regardless of adjunctive therapies. It offers improved efficacy at tumor eradication, reduced recurrences, and the potential to spare the native bladder. This systematic review serves as a call to action for better designed clinical trials, and indeed, there are 109 ongoing clinical trials involving some form of hyperthermia [42]. The promise of deep regional hyperthermia seen by the effect size has been largely ignored secondary to the disparate and small body of evidence. This nascent technology has been smoldering for years, and is now ready to be delivered to the forefront of therapy, particularly for high risk NMIBC, and for those patients not suitable for a cystectomy.
Figure 1.
Flow diagram of evidence acquisition
Table 2.
Overview of toxicity associated with regional hyperthermia therapy
| Study | General | Fatigue | Myalgia | Anxiety | Genitourinary | Urethral pain | Cystitis | Dysuria | Hematuria | Bladder spasm | Weak urinary stream | Vaginitis | Frequency | Contracted bladder | Incontinence |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Geijsen et al | 10 (7%) | ||||||||||||||
| Hisazumi et al | |||||||||||||||
| Inman et al | 2 (13%) | 1 (7%) | 1 (7%) | 6 (40%) | 4 (27%) | 3 (20%) | 2 (13%) | 1 (7%) | 1 (7%) | ||||||
| Masunaga et al | |||||||||||||||
| Nishimura et al * | 1 (3%) | ||||||||||||||
| Noguchi et al | 24 (69%) | ||||||||||||||
| Ohguri et al | 3 (100%) | ||||||||||||||
| Petrovich et al * | |||||||||||||||
| Rietbroek et al | |||||||||||||||
| Sapozink et al | 11 (26%) | 6 (14%) | 11 (26%) | ||||||||||||
| Uchibayashi et al | |||||||||||||||
| Ueda et al | 3 (27%) | ||||||||||||||
| van der Zee et al * | |||||||||||||||
| Wittlinger et al. | 7 (16%) | 1 (2%) |
| Dermatologic | Burns/blister | Induration/ swelling | Pruitis | Rash | Bruising | Flushing | Fat necrosis | Neurologic | Dizziness | Confusion | Extrapyramidal sympoms | Infectious | Urinary tract infection | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Geijsen et al | ||||||||||||||
| Hisazumi et al | 21/39 (54%) | 10/39 (26%) | ||||||||||||
| Inman et al | 4 (27%) | 3 (20%) | 2 (13%) | 1 (7%) | 1 (7%) | 2 (13%) | 1 (7%) | 1 (7%) | 2 (13%) | |||||
| Masunaga et al | ||||||||||||||
| Nishimura et al * | 3 (10%) | 2 (6%) | ||||||||||||
| Noguchi et al | ||||||||||||||
| Ohguri et al | ||||||||||||||
| Petrovich et al * | 4 (8%) | |||||||||||||
| Rietbroek et al | 2 (14%) | 5 (36%) | ||||||||||||
| Sapozink et al | 2 (5%) | |||||||||||||
| Uchibayashi et al | 15 (33%) | 9 (20%) | ||||||||||||
| Ueda et al | ||||||||||||||
| van der Zee et al * | 5 (3%) | 20 (11%) | ||||||||||||
| Wittlinger et al. |
| Cardiovascular | Hypertension | Gastrointestinal | Nausea or vomiting | Anorexia | Constipation | Ileus | Diarrhea | Abdominal pain | Procedure related | Positional discomfort | Heat intolerance/pain with applicator | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Geijsen et al | 22 (16%) | 29 (20%) | ||||||||||
| Hisazumi et al | ||||||||||||
| Inman et al | 3 (20%) | 2 (13%) | 1 (7%) | 5 (33%) | 1 (7%) | |||||||
| Masunaga et al | 0 (0%) | 1 (4%) | 12 (43%) | |||||||||
| Nishimura et al * | 18 (55%) | |||||||||||
| Noguchi et al | 17 (49%) | 4 (11%) | ||||||||||
| Ohguri et al | 1 (33%) | |||||||||||
| Petrovich et al * | 4 (8%) | 25 (47%) | ||||||||||
| Rietbroek et al | 31% of treat-ments | |||||||||||
| Sapozink et al | 8 (19%) | 11 (24%) | 32 (74%) | |||||||||
| Uchibayashi et al | ||||||||||||
| Ueda et al | ||||||||||||
| van der Zee et al * | ||||||||||||
| Wittlinger et al. | 1 (2%) | 1 (2%) |
Footnotes
PROSPERO Registration number: CRD42015025780
Declaration of interests
The authors report no declarations of interest.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: A Cancer Journal for Clinicians. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Burger M, Catto JW, Dalbagni G, Grossman HB, Herr H, Karakiewicz P, et al. Epidemiology and risk factors of urothelial bladder cancer. European urology. 2013;63:234–41. doi: 10.1016/j.eururo.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 3.Cambier S, Sylvester RJ, Collette L, Gontero P, Brausi MA, van Andel G, et al. EORTC Nomograms and Risk Groups for Predicting Recurrence, Progression, and Disease-specific and Overall Survival in Non-Muscle-invasive Stage Ta-T1 Urothelial Bladder Cancer Patients Treated with 1–3 Years of Maintenance Bacillus Calmette-Guerin. European urology. 2015 doi: 10.1016/j.eururo.2015.06.045. [DOI] [PubMed] [Google Scholar]
- 4.The National Comprehensive Cancer Network. Bladder Cancer, version 2.20125. [Google Scholar]
- 5.Hall MC, Chang SS, Dalbagni G, Pruthi RS, Seigne JD, Skinner EC, et al. Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. The Journal of urology. 2007;178:2314–30. doi: 10.1016/j.juro.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Botteman MF, Pashos CL, Redaelli A, Laskin B, Hauser R. The health economics of bladder cancer: a comprehensive review of the published literature. PharmacoEconomics. 2003;21:1315–30. doi: 10.1007/BF03262330. [DOI] [PubMed] [Google Scholar]
- 7.Frey B, Weiss EM, Rubner Y, Wunderlich R, Ott OJ, Sauer R, et al. Old and new facts about hyperthermia-induced modulations of the immune system. International Journal of Hyperthermia. 2012;28(6) doi: 10.3109/02656736.2012.677933. [DOI] [PubMed] [Google Scholar]
- 8.Toraya-Brown S, Fiering S. Local tumour hyperthermia as immunotherapy for metastatic cancer. International journal of hyperthermia : the official journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group. 2014;30:531–9. doi: 10.3109/02656736.2014.968640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engelhardt R. Rational for clinical application of hyperthermia and drugs. Revista medico-chirurgicala a Societatii de Medici si Naturalisti din Iasi. 1987;91:347–52. [PubMed] [Google Scholar]
- 10.Gofrit ON, Shapiro A, Pode D, Sidi A, Nativ O, Leib Z, et al. Combined local bladder hyperthermia and intravesical chemotherapy for the treatment of high-grade superficial bladder cancer. Urology. 2004;63:466–71. doi: 10.1016/j.urology.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 11.Wallner KE, Banda M, Li GC. Hyperthermic enhancement of cell killing by mitomycin C in mitomycin C-resistant Chinese hamster ovary cells. Cancer research. 1987;47:1308–12. [PubMed] [Google Scholar]
- 12.van der Heijden AG, Jansen CF, Verhaegh G, O’Donnell MA, Schalken JA, Witjes JA. The effect of hyperthermia on mitomycin-C induced cytotoxicity in four human bladder cancer cell lines. European urology. 2004;46:670–4. doi: 10.1016/j.eururo.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 13.Colombo R, Salonia A, Leib Z, Pavone-Macaluso M, Engelstein D. Long-term outcomes of a randomized controlled trial comparing thermochemotherapy with mitomycin-C alone as adjuvant treatment for non-muscle-invasive bladder cancer (NMIBC) BJU international. 2011;107:912–8. doi: 10.1111/j.1464-410X.2010.09654.x. [DOI] [PubMed] [Google Scholar]
- 14.Rigatti P, Lev A, Colombo R. Combined intravesical chemotherapy with mitomycin C and local bladder microwave-induced hyperthermia as a preoperative therapy for superficial bladder tumors. A preliminary clinical study. European urology. 1991;20:204–10. doi: 10.1159/000471701. [DOI] [PubMed] [Google Scholar]
- 15.Sousa A, Inman BA, Pineiro I, Monserrat V, Perez A, Aparici V, et al. A clinical trial of neoadjuvant hyperthermic intravesical chemotherapy (HIVEC) for treating intermediate and high-risk non-muscle invasive bladder cancer. International journal of hyperthermia : the official journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group. 2014;30:166–70. doi: 10.3109/02656736.2014.900194. [DOI] [PubMed] [Google Scholar]
- 16.Oliveira TR, Stauffer PR, Lee CT, Landon CD, Etienne W, Ashcraft KA, et al. Magnetic fluid hyperthermia for bladder cancer: a preclinical dosimetry study. International journal of hyperthermia : the official journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group. 2013;29:835–44. doi: 10.3109/02656736.2013.834384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Zee J, Gonzalez Gonzalez D, van Rhoon GC, van Dijk JD, van Putten WL, Hart AA. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: a prospective, randomised, multicentre trial. Dutch Deep Hyperthermia Group. Lancet (London, England) 2000;355:1119–25. doi: 10.1016/s0140-6736(00)02059-6. [DOI] [PubMed] [Google Scholar]
- 18.Lammers RJ, Witjes JA, Inman BA, Leibovitch I, Laufer M, Nativ O, et al. The role of a combined regimen with intravesical chemotherapy and hyperthermia in the management of non-muscle-invasive bladder cancer: a systematic review. European urology. 2011;60:81–93. doi: 10.1016/j.eururo.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 19.Yuan Y, Cheng KS, Craciunescu OI, Stauffer PR, Maccarini PF, Arunachalam K, et al. Utility of treatment planning for thermochemotherapy treatment of nonmuscle invasive bladder carcinoma. Medical physics. 2012;39:1170–81. doi: 10.1118/1.3679839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wittlinger M, Rodel CM, Weiss C, Krause SF, Kuhn R, Fietkau R, et al. Quadrimodal treatment of high-risk T1 and T2 bladder cancer: transurethral tumor resection followed by concurrent radiochemotherapy and regional deep hyperthermia. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2009;93:358–63. doi: 10.1016/j.radonc.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 21.Inman BA, Stauffer PR, Craciunescu OA, Maccarini PF, Dewhirst MW, Vujaskovic Z. A pilot clinical trial of intravesical mitomycin-C and external deep pelvic hyperthermia for non-muscle-invasive bladder cancer. International journal of hyperthermia : the official journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group. 2014;30:171–5. doi: 10.3109/02656736.2014.882021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petrovich Z, Emami B, Kapp D, Sapozink MD, Langholz B, Oleson J, et al. Regional hyperthermia in patients with recurrent genitourinary cancer. American journal of clinical oncology. 1991;14:472–7. doi: 10.1097/00000421-199112000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Sapozink MD, Gibbs FA, Jr, Egger MJ, Stewart JR. Regional hyperthermia for clinically advanced deep-seated pelvic malignancy. American journal of clinical oncology. 1986;9:162–9. doi: 10.1097/00000421-198604000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Juang T, Stauffer PR, Craciunescu OA, Maccarini PF, Yuan Y, Das SK, et al. Thermal dosimetry characteristics of deep regional heating of non-muscle invasive bladder cancer. International journal of hyperthermia : the official journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group. 2014;30:176–83. doi: 10.3109/02656736.2014.898338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crezee J, Van Haaren PM, Westendorp H, De Greef M, Kok HP, Wiersma J, et al. Improving locoregional hyperthermia delivery using the 3-D controlled AMC-8 phased array hyperthermia system: a preclinical study. International journal of hyperthermia : the official journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group. 2009;25:581–92. doi: 10.3109/02656730903213374. [DOI] [PubMed] [Google Scholar]
- 26.Kok HP, Ciampa S, de Kroon-Oldenhof R, Steggerda-Carvalho EJ, van Stam G, Zum Vorde Sive Vording PJ, et al. Toward online adaptive hyperthermia treatment planning: correlation between measured and simulated specific absorption rate changes caused by phase steering in patients. International journal of radiation oncology, biology, physics. 2014;90:438–45. doi: 10.1016/j.ijrobp.2014.05.1307. [DOI] [PubMed] [Google Scholar]
- 27.Geijsen ED, de Reijke TM, Koning CC, Zum Vorde Sive Vording PJ, de la Rosette JJ, Rasch CR, et al. Combining Mitomycin C and Regional 70 MHz Hyperthermia in Patients with Nonmuscle Invasive Bladder Cancer: A Pilot Study. The Journal of urology. 2015 doi: 10.1016/j.juro.2015.05.102. [DOI] [PubMed] [Google Scholar]
- 28.Rietbroek RC, Bakker PJ, Schilthuis MS, Postma AJ, Zum Vorde Sive Vording PJ, Gonzalez Gonzalez D, et al. Feasibility, toxicity, and preliminary results of weekly loco-regional hyperthermia and cisplatin in patients with previously irradiated recurrent cervical carcinoma or locally advanced bladder cancer. International journal of radiation oncology, biology, physics. 1996;34:887–93. doi: 10.1016/0360-3016(95)02152-3. [DOI] [PubMed] [Google Scholar]
- 29.Lee CK, Song CW, Rhee JG, Foy JA, Levitt SH. Clinical experience using 8 MHz radiofrequency capacitive hyperthermia in combination with radiotherapy: results of a phase I/II study. International journal of radiation oncology, biology, physics. 1995;32:733–45. doi: 10.1016/0360-3016(94)00608-N. [DOI] [PubMed] [Google Scholar]
- 30.Hiraoka M, Jo S, Akuta K, Nishimura Y, Nagata Y, Takahashi M, et al. Clinical results of radiofrequency capacitive hyperthermia in deep-seated tumors. Gan no rinsho Japan journal of cancer clinics. 1986;32:1679–84. [PubMed] [Google Scholar]
- 31.Sackett DL, Rosenberg WM, Gray JA, Haynes RB, Richardson WS. Evidence based medicine: what it is and what it isn’t. 1996. Clinical orthopaedics and related research. 2007;455:3–5. [PubMed] [Google Scholar]
- 32.Ueda K, Sakagami H, Masui Y, Okamura T. Single instillation of hydroxypropylcellulose-doxorubicin as treatment for superficial bladder carcinoma. Cancer chemotherapy and pharmacology. 1994;35(Suppl):S81–3. doi: 10.1007/BF00686926. [DOI] [PubMed] [Google Scholar]
- 33.Noguchi S, Kubota Y, Miura T, Shuin T, Hosaka M. Use of methotrexate, vinblastine, adriamycin, and cisplatin in combination with radiation and hyperthermia as neo-adjuvant therapy for bladder cancer. Cancer chemotherapy and pharmacology. 1992;30(Suppl):S63–5. doi: 10.1007/BF00686945. [DOI] [PubMed] [Google Scholar]
- 34.Hisazumi H, Nakajima K. Eight-MHZ RF hyperthermia in urological malignancies. Gan to kagaku ryoho Cancer & chemotherapy. 1988;15:1382–6. [PubMed] [Google Scholar]
- 35.Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Seminars in radiation oncology. 2003;13:176–81. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 36.Naito K, Hasegawa T, Ishida T, Yamamoto H, Mihara S, Komatsu K, et al. A clinical survey of advanced bladder cancer: treatment of advanced and non-resectable bladder cancer. Hinyokika kiyo Acta urologica Japonica. 1991;37:1601–6. [PubMed] [Google Scholar]
- 37.Masunaga SI, Hiraoka M, Akuta K, Nishimura Y, Nagata Y, Jo S, et al. Phase I/II trial of preoperative thermoradiotherapy in the treatment of urinary bladder cancer. International journal of hyperthermia. 1994:31–40. doi: 10.3109/02656739409009329. [DOI] [PubMed] [Google Scholar]
- 38.Uchibayashi T, Yamamoto H, Kunimi K, Koshida K, Nakajima K. Radiofrequency capacitive hyperthermia combined with irradiation or chemotherapy for patients with invasive bladder cancers. International urology and nephrology. 1995;27:735–41. doi: 10.1007/BF02552139. [DOI] [PubMed] [Google Scholar]
- 39.Nishimura Y, Hiraoka M, Jo S, Akuta K, Nagata Y, Masunaga S, et al. Radiofrequency (RF) capacitive hyperthermia combined with radiotherapy in the treatment of abdominal and pelvic deep-seated tumors. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 1989;16:139–49. doi: 10.1016/0167-8140(89)90031-5. [DOI] [PubMed] [Google Scholar]
- 40.Ohguri T, Imada H, Nomoto S, Kato F, Yahara K, Morioka T, et al. Initial Experience of Bladder Preservation Therapy Using Chemoradiotherapy with Regional Hyperthermia for Muscle-invasive Bladder Cancer. Thermal Medicine. 2005;21:151–8. [Google Scholar]
- 41.Ip S, Paulus JK, Balk EM, Dahabreh IJ, Avendano EE, Lau J. Role of Single Group Studies in Agency for Healthcare Research and Quality Comparative Effectiveness Reviews. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013. AHRQ Methods for Effective Health Care. [PubMed] [Google Scholar]
- 42.Cihoric N, Tsikkinis A, van Rhoon G, Crezee H, Aebersold DM, Bodis S, et al. Hyperthermia-related clinical trials on cancer treatment within the ClinicalTrials.gov registry. International journal of hyperthermia : the official journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group. 2015;31:609–14. doi: 10.3109/02656736.2015.1040471. [DOI] [PubMed] [Google Scholar]