Figure 1. Two key ER quality control systems in mammals.
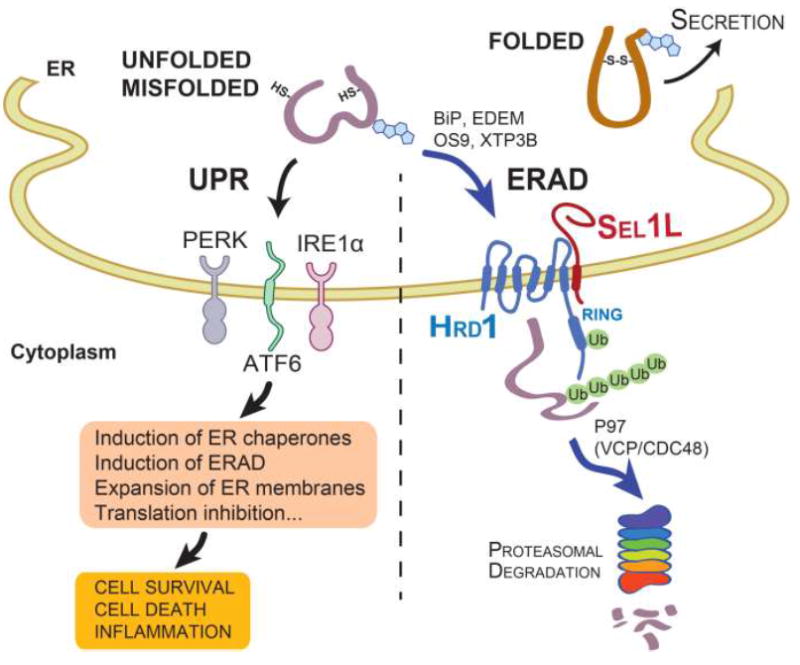
While folded proteins exit the ER, terminally unfolded or misfolded proteins in the ER activate UPR via three sensors IRE1α, PERK and ATF6, which initiate multiple signaling pathways leading to the induction of ER chaperones and ERAD, expansion of ER membranes and translation inhibition. In addition, misfolded proteins can be recruited to the ERAD complex via the activity of various ER chaperones such as BiP, EDEM, OS9 and XTP3B for cytosolic degradation. The Sel1L-Hrd1 protein complex represents the most conserved ERAD complex in mammals. Following retrotranslocation into the cytosol, substrates are ubiquitinated and, with the help of p97 (VCP/CDC48), degraded by the proteasome in the cytosol.
