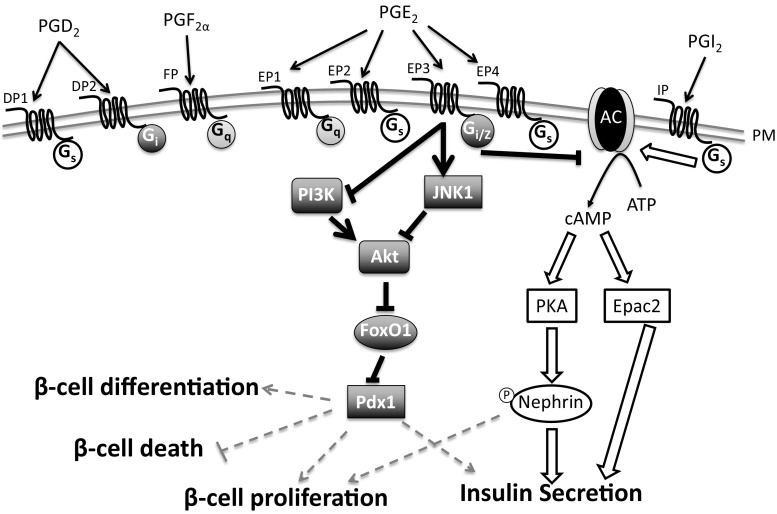
Fig. 3.
Summary of PG receptor signaling. A schematic of some of the proposed signaling mechanisms of PG receptors in the β-cell. Currently, there are no demonstrated effects of DP1, DP2, FP, or EP1 in regulating either β-cell function or mass. EP2 and EP4 improve glucose-stimulated insulin secretion (GSIS) in vivo, but no mechanism has been determined. EP3 couples to Gi proteins, including GaZ, which inhibit adenylyl cyclase (AC) and reduce islet cAMP levels. However, it is unclear if this is the mechanism responsible for alterations in GSIS and β-cell mass dynamics. In cell lines and rodent islets, EP3 signaling can either inhibit phosphatidylinositol 3-kinase (PI3K) or activate c-Jun N-terminal kinase (JNK), which leads to dephosphorylation and inhibition of Akt. Akt normally phosphorylates and inhibits forkhead box O1 (FoxO1), so upon EP3 signaling, FoxO1 is dephosphorylated and undergoes nuclear translocation. Pancreatic and duodenal homeobox-1 (Pdx1) is important in regulating GSIS, β-cell differentiation, and β-cell mass dynamics, such as proliferation and cell death. FoxO1 and Pdx1 are mutually exclusive in the nucleus, thus providing a potential mechanism for EP3-induced decreases in GSIS and β-cell proliferation. PGI2 via IP signaling results in GS-mediated activation of AC and increased islet cAMP levels. cAMP activates protein kinase A (PKA) or Epac2. In β-cell lines, IP signaling increases GSIS by either PKA activation and phosphorylation of Nephrin or by Epac2 signaling. The IP-induced downstream targets of Epac2 that increase GSIS have not been demonstrated. Dark gray symbols and bold lines indicate PGE2-EP3 signaling pathways. Outlined symbols and block arrows indicate PGI2-IP signaling pathways. Dashed gray symbols represent potential but not confirmed events