Abstract
Localized cutaneous nodular amyloidosis (LCNA) is the rarest form of localized cutaneous amyloidosis. In patients with LCNA, local plasma cells secrete immunoglobulin light chains called amyloid L via an unknown mechanism. LCNA has been associated with autoimmune connective tissue diseases such as most commonly Sjögren syndrome. A few reported cases of LCNA are associated with limited systemic sclerosis (LSSc). We report three cases of LCNA in patients with LSSc to add to the existing literature, discuss the disease association and proposed pathophysiology, and briefly review the existing information in current literature. It is important to closely follow patients with LCNA to monitor progression to systemic amyloidosis.
Keywords: nodular amyloidosis, limited systemic sclerosis, connective tissue disease, histopathology
Introduction
Localized cutaneous nodular amyloidosis (LCNA) is the rarest form of localized cutaneous amyloidosis (Summers and Kendrick 2008) and may be associated with autoimmune connective tissue diseases such as Sjögren syndrome (SS; Meijer et al. 2008) and limited systemic sclerosis (LSSc; formerly known as CREST syndrome; Damian and Bertouch, 2010, Shiman et al., 2010, Summers and Kendrick, 2008). We report three cases of LCNA in patients with LSSc and briefly review existing information in current literature.
Report of Three Cases
Case 1
A 71-year-old woman with a 4-year history of LSSc presented with tender nodules and plaques on the leg for the last year. A physical examination revealed 2 to 4 cm, skin-colored, hyperpigmented, and firm plaques and a skin-colored nodule on the left lower leg (Fig. 1). Sclerodactyly and scattered mat telangiectasias were also noted. The patient was taking omeprazole for esophageal dysmotility. A histopathologic examination demonstrated scattered plasma cells and amorphous pink material in the dermis and subcutis (Fig. 2) that was apple-green birefringent with Congo-red staining and polarized light (Figs. 3 and 4), which is consistent with nodular amyloidosis. Laboratory testing for systemic disease included a normal metabolic panel, complete blood count, and serum and urine protein electrophoresis. Prior testing included an unremarkable echocardiogram and pulmonary function test and a positive antinuclear antibodies (ANA) test of > 1:2560 with a centromere pattern that is consistent with LSSc. The patient elected to be treated with intralesional triamcinolone (10 mg/ml), one injection per month for 3 months, which resulted in a modest improvement. Serum protein electrophoresis remained normal 1 year later and the patient is scheduled to be re-evaluated annually.
Fig. 1.
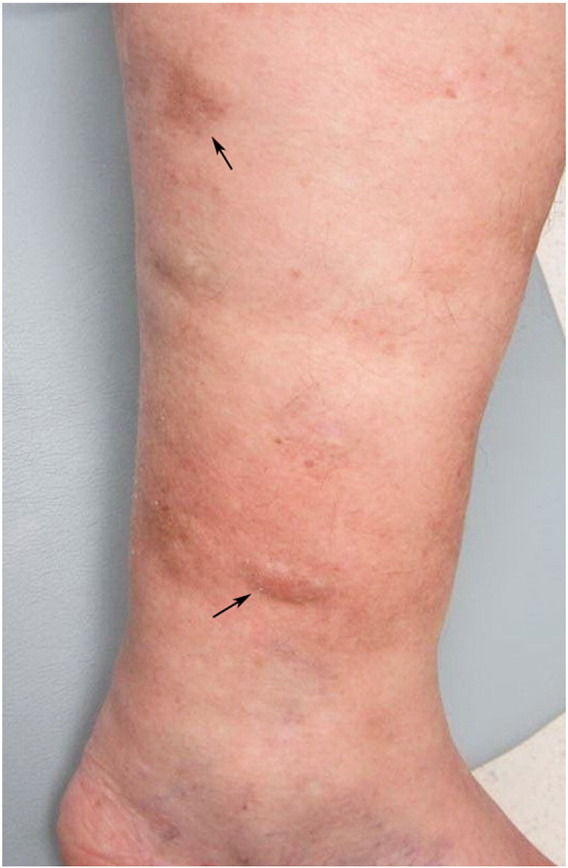
Clinical photograph of case 1 demonstrating skin-colored to brown, subcutaneous nodules and firm plaques on the left lower leg.
Fig. 2.
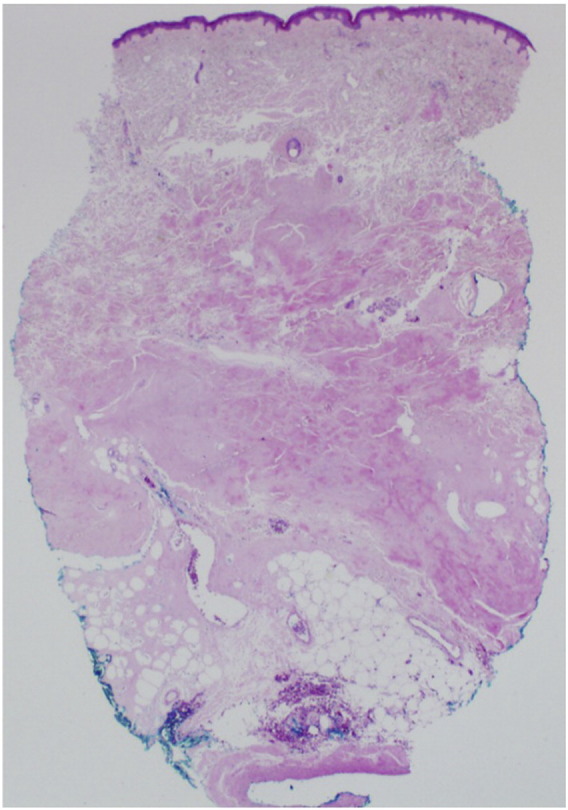
Histopathologic image of a skin biopsy specimen from a subcutaneous nodule on the left lower leg of patient 1 demonstrating mid-to-deep dermis that is filled with pink amorphous material (hematoxylin-eosin, original magnification x2.5).
Fig. 3.
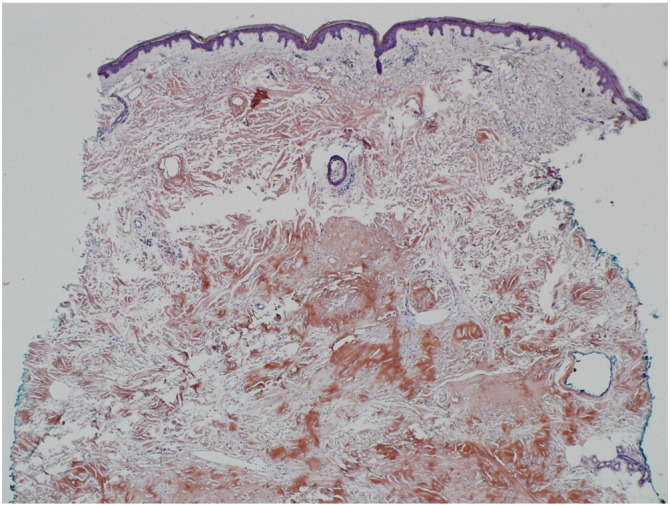
Histopathologic image of a skin biopsy specimen from a subcutaneous nodule on the left lower leg of patient 1 stained with Congo red (original magnification x4).
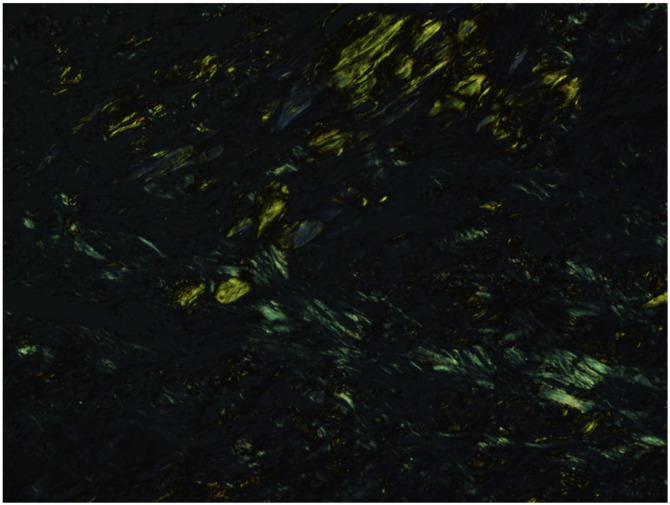
Fig. 4.
Histopathologic image of a skin biopsy specimen from a subcutaneous nodule on the left lower leg of patient 1 under polarized light demonstrating apple-green birefringence (original magnification x10).
Case 2
A 58-year-old woman with a 4-year history of LSSc presented with painful shin nodules (Fig. 5) that had developed over the preceding 18 months. An examination of skin biopsy tissue revealed amorphous pink material with perivascular accentuation and scattered plasma cells. The material showed strongly-positive fluorescence with thioflavin T staining that is consistent with nodular amyloidosis. Serum immunofixation electrophoresis revealed a faint monoclonal kappa light chain band with slightly elevated immunoglobulin (Ig) G and IgM levels. The test results of the bone marrow biopsy tissue were unremarkable. Lung nodules were found only incidentally and biopsy tissue tests showed amyloidosis. The results of monitoring echocardiograms were within the normal limits. Intralesional triamcinolone was selected to treat the leg nodules.
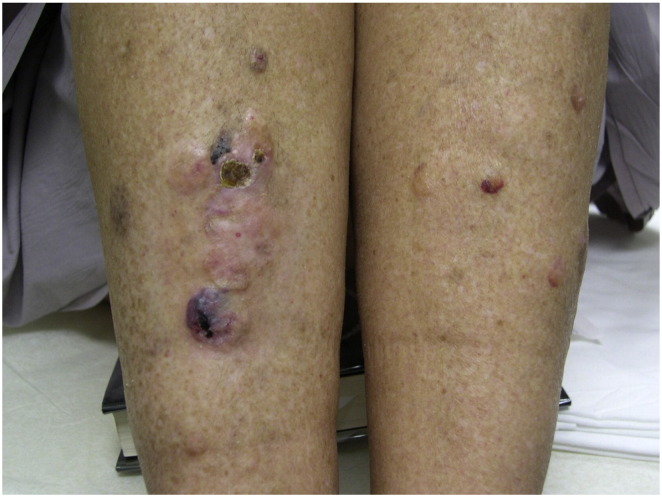
Fig. 5.
Clinical photograph of case 2 demonstrating violaceous to skin-colored nodules on the lower legs with overlying ulceration in areas.
Triamcinolone (10 mg/ml) was injected once a month for 4 months and result in a modest improvement. Five years after the diagnosis of LCNA, the patient was diagnosed with SS on the basis of xerostomia and xerophthalmia symptoms and a positive SSA antibody test result. Her monoclonal gammopathy remained stable over a period of 9 years.
Case 3
A 70-year-old female patient with a 22-year history of LSSc presented with a tender nodule on the shin that was first noted 4 to 5 years prior. A physical examination demonstrated a 3.5 cm, skin-colored, focally ulcerated pink nodule with a firm immobile subcutaneous and softer superficial portion with three associated flesh-colored plaques (Fig. 6). Sclerodactyly, calcinosis cutis that involves the forearm, decreased oral aperture, and facial and chest telangiectasias were also noted during the physical examination. Treatments for LSSc included amlodipine, omeprazole, and physical therapy. An analysis of biopsy tissue from the shin revealed amorphous pink material that highlighted with Congo-red staining with admixed sparse chronic inflammation that is consistent with nodular amyloidosis. Other notable test results included ANA levels of 1:640 with a centromere pattern, leukopenia, and subclinical hypothyroidism. Results from a metabolic panel, serum and urine electrophoresis, pulmonary function testing, and an echocardiogram were within normal limits. The patient’s large nodule was surgically debulked with subsequent local wound care dressings. At the time of the follow-up examination 1 year later, the primary nodule showed resolution with improvement in tenderness and a secondary nodule showed newly-developed, superficial ulceration (Fig. 7).
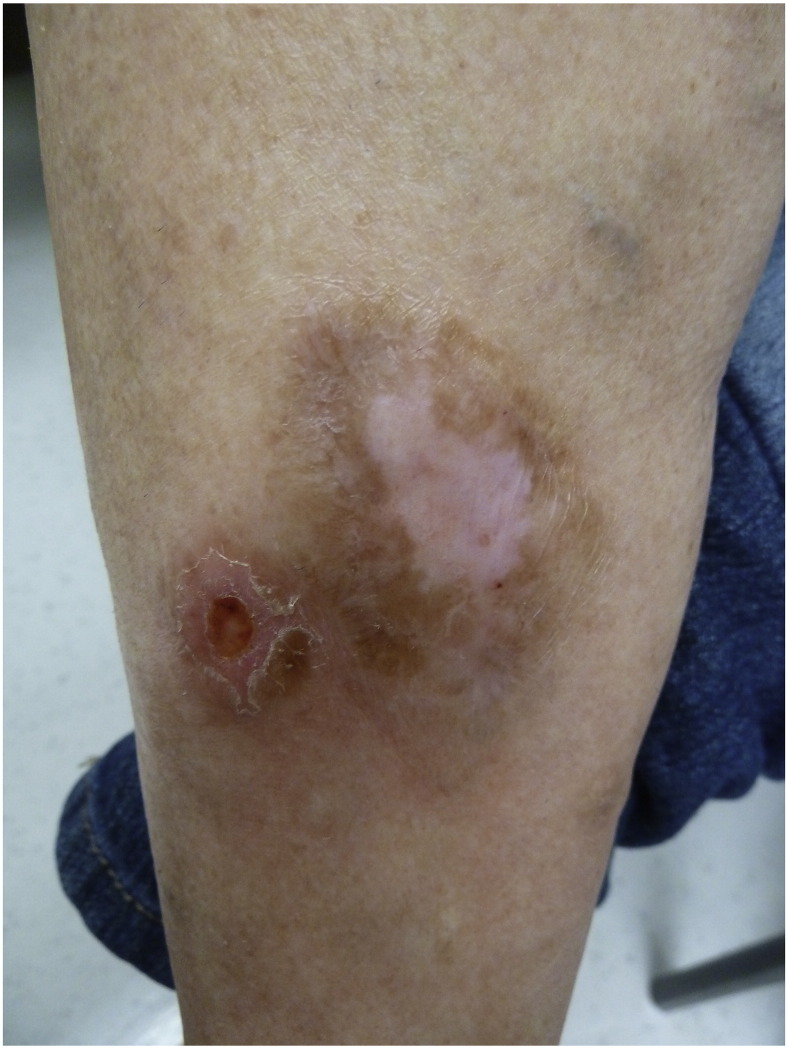
Fig. 6.
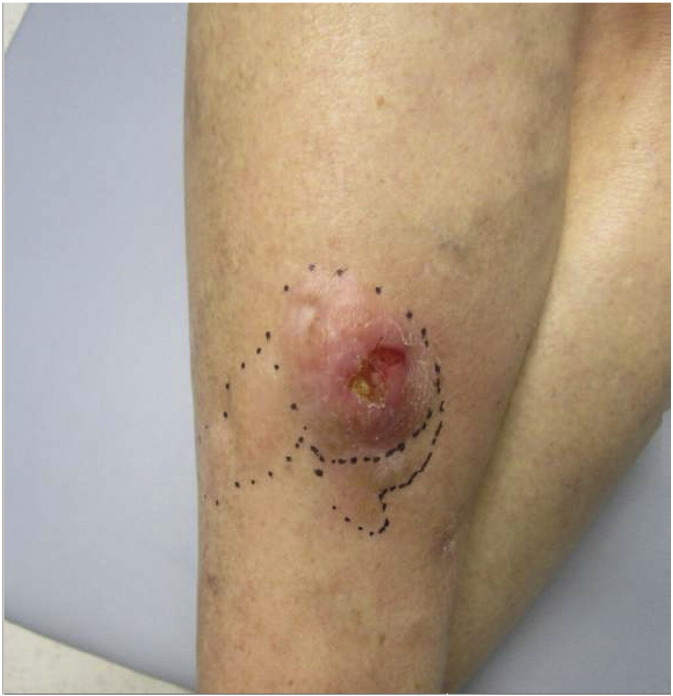
Clinical photograph of case 3 demonstrating an ulcerated firm nodule on the right lower leg.
Fig. 7.
Clinical photograph of case 3 taken at 1-year post-surgical debulking.
Discussion
There are three previously reported cases of LCNA in the setting of LSSc (Damian and Bertouch, 2010, Shiman et al., 2010, Summers and Kendrick, 2008). The features of the current and the previously reported cases are summarized in Table 1. In the cases that are presented in this study, all three patients had a diagnosis of LSSc when they presented with LCNA. In all reported cases, patients ranged in age from 58 to 83 years, were predominately female, and presented only with LCNA involvement of the unilateral or bilateral lower extremities (Damian and Bertouch, 2010, Meijer et al., 2008, Shiman et al., 2010, Summers and Kendrick, 2008). The follow-up time ranged from 1 to 9 years and no patient developed systemic amyloidosis.
Table 1.
Summary of case features of localized cutaneous nodular amyloidosis in limited systemic sclerosis.
| Reference | Age, yrs | Sex | Length of LSSc Diagnosis (yrs) | Location of LCNA | yrs nodules present before presentation, progression | LCNA Treatments Tried, Results | LSSc Therapy | Negative systemic workup | Positive systemic workup | Follow up (yrs) |
|---|---|---|---|---|---|---|---|---|---|---|
| Summers and Kendrick, 2008 | 61 | Female | 1 | left shin | unknown, increasing in number and extending to bilateral lower extremities over the next 3 years | pulsed dye laser every 6 weeks, improved tenderness of lesions | unknown | CBC, glucose, serum-free light chains, paraproteinemia, SPEP/UPEP, RFT, echo, chest CT, skeletal survey, bone marrow biopsy | elevated LFT secondary to fatty liver | 8 |
| Shiman et al. 2010 | 83 | Female | 0 | bilateral lower extremities | 25, increasing in size and number over this time | unknown | unknown | unknown | unknown | unknown |
| Damian et al. 2010 | 61 | Male | 10 | bilateral lower extremities | 1.5 | patient declined therapy | unknown | CBC, serum free light chains, serum immunoelectrophroretogram, SPEP/UPEP, UA, LFT, RFT, TFT, echo, chest CT, abdomen CT, pelvis CT, abdominal biopsy, rectal biopsy | none | unknown |
| Current study | 71 | Female | 4 | left shin | 1 | monthly intralesional triamcinolone (10 mg/ml) injections over 3 months, modest improvement | omeprazole for esophageal dysmotility | CBC, BMP, SPEP/UPEP, PFT, echo | none | 1 |
| Current study | 58 | Female | 4 | bilateral lower extremities | 1.5 | monthly intralesional triamcinolone (10 mg/ml) injections over 4 months, modest improvement | unknown | echo, bone marrow biopsy | serum immunofixation electrophoresis, lung nodules of nodular amyloidosis | 9, diagnosed with Sjögren Syndrome at year 5 |
| Current study | 70 | Female | 22 | right shin | 4-5, enlarging over this time | surgically debulked, resolution and improved tenderness | amlodipine for Raynaud phenomenon, omeprazole for esophageal dysmotility, physical therapy for sclerodactyly | BMP, SPEP/UPEP, PFT, echo | leukopenia, subclinical hypothyroidism | 1 |
BMP: basic metabolic panel; CBC: complete blood count; CT: computed tomography; LCNA: localized cutaneous nodular amyloidosis; LFT: liver function tests; LSSc: limited systemic sclerosis; PFT: pulmonary function tests; RFT: renal function tests; SPEP/UPEP: serum protein electrophoresis/urine protein electrophoresis; TFT: thyroid function tests; echo: echocardiogram; UA: urinalysis.
Typically, LCNA is caused by the dermal deposition of Ig light chains that are produced by locally-infiltrating plasma cells via an unclear mechanism (Summers and Kendrick 2008). Light chains may be λ, κ, or both (Woollons and Black 2001). Amyloid L is the same type of amyloid that is involved in primary systemic and multiple-myeloma-associated systemic amyloidosis (Summers and Kendrick 2008). However, there has been report of keratinocyte-derived amyloid (AK) that is implicated in LCNA (Cornejo et al. 2015). Cutaneous macular and lichen amyloidoses are composed of amyloid K (Shiman et al. 2010).
Clinically, the cutaneous findings of LCNA and primary systemic amyloidosis can be identical and monitoring patients longitudinally is important because approximately 7% of patients with LCNA progress to systemic amyloidosis (Woollons and Black 2001). To our knowledge, none of the six patients who reported with LCNA developed systemic disease. Additionally, there is an association between LCNA and autoimmune connective tissue diseases and 25% of reported cases are diagnosed with SS (Meijer et al. 2008). The postulated link between SS and LCNA is light chain-producing plasma cells that play a role in the pathogenesis of both entities (Meijer et al. 2008). We hypothesize that the same could be true for LSSc. Others have hypothesized that LSSc may alter growth factors and cytokines that impair normal plasma cell function (Shiman et al. 2010). Additionally, one could hypothesize that therapies to treat existing autoimmune connective tissue disease may lead to the development of LCNA but no data is available to support this hypothesis.
Localized nodular amyloidosis has been reported in the breasts and lungs of patients with SS (Meijer et al. 2008). It is currently unclear whether multifocal localized nodular amyloidosis in the skin, breast, and lung could be a distinct clinical entity in patients with SS. The term SS-associated localized nodular amyloidosis (SALNA) has been proposed to describe this entity (Meijer et al. 2008). Even though the second patient we report developed SS, the finding of focal nodular amyloidosis in this patient’s skin and lung is especially of interest because this has not been previously reported in a patient with LSSc. There is no clear explanation why LCNA lesions prefer the lower extremities of patients. Because the lower leg is a trauma-prone area, one may infer that the nodules form in response to tissue insult. However, no adequate evidence exists to support this.
Treatment of LCNA is difficult and many nodules recur after treatment. In cases 1 and 2, intralesional triamcinolone (10 mg/ml) resulted in a modest improvement but case 3 elected the shave debulking procedure. The debulking procedure resulted in resolution of the nodule and improvement in tenderness. In the previously-reported cases, one patient underwent pulsed dye laser every 6 weeks for an unspecified amount of time to treat the nodules and reported improvement in tenderness (Summers and Kendrick 2008). Another patient declined therapy, citing that the lesions were asymptomatic and of no cosmetic concern (Damian and Bertouch 2010). It is unknown what, if any, treatment the other patient underwent (Shiman et al. 2010). Other reported treatment modalities include surgical excision, systemic steroids, curettage, dermabrasion, and pulsed dye laser, which all have mixed results (Damian and Bertouch, 2010, Meijer et al., 2008, Woollons and Black, 2001). One could hypothesize that with continued control of LSSc, progression to LCNA may be avoided but further investigation is required to study this association. We present these cases to highlight the association between LCNA and connective tissue diseases and particularly LSSc.
Footnotes
Funding sources: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Cornejo K.M., Lagana F.J., Deng A. Nodular amyloidosis derived from keratinocytes: an unusual type of primary localized cutaneous nodular amyloidosis. Am J Dermatopathol. 2015;37(11):e129–e133. doi: 10.1097/DAD.0000000000000307. [DOI] [PubMed] [Google Scholar]
- Damian D.L., Bertouch J.V. Images in dermatology. A plethora of protein. Primary localized cutaneous nodular amyloidosis. Am J Med. 2010;123(10):904–906. doi: 10.1016/j.amjmed.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Meijer J.M., Schonland S.O., Palladini G., Merlini G., Hegenbart U., Ciocca O. Sjögren's Syndrome and localized nodular cutaneous amyloidosis: coincidence or a distinct clinical entity? Arthritis Rheum. 2008;58(7):1992–1999. doi: 10.1002/art.23617. [DOI] [PubMed] [Google Scholar]
- Shiman M., Ricotti C., Miteva M., Kerdel F., Romanelli P. Primary localized cutaneous nodular amyloidosis associated with CREST syndrome. Int J Dermatol. 2010;49(2):229–230. doi: 10.1111/j.1365-4632.2008.04058.x. [DOI] [PubMed] [Google Scholar]
- Summers E.M., Kendrick C.G. Primary localized cutaneous nodular amyloidosis and CREST syndrome: A case report and review of the literature. Cutis. 2008;82(1):55–59. [PubMed] [Google Scholar]
- Woollons A., Black M.M. Nodular localized primary cutaneous amyloidosis: A long-term follow-up study. Br J Dermatol. 2001;145(1):105–109. doi: 10.1046/j.1365-2133.2001.04291.x. [DOI] [PubMed] [Google Scholar]