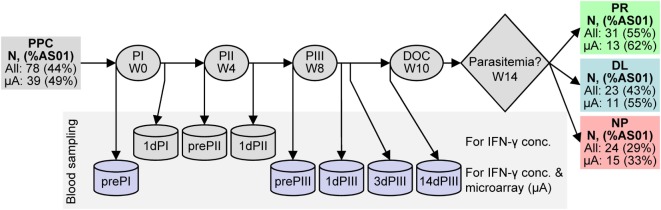
Figure 1.
Schematic representation of RTS,S candidate vaccine clinical trial design and efficacy results. RTS,S vaccine injections were performed at weeks (W) 0, 4, and 8. Blood was sampled (represented by can shapes) before the first, second, and third vaccine injections (prePI, prePII, and prePIII, respectively), one day after the first, second, and third vaccine injections (1dPI, 1dPII, and 1dPIII, respectively), and 3 and 14 days after the third vaccine injection (3dPIII, 10dPIII, and 14dPIII, respectively). Controlled human malaria infection (CHMI) was performed at 14dPIII (i.e., week 10; day of challenge, DOC) and the onset of parasitemia was followed up to week 14. Serum IFN-γ concentrations were measured at all of the time points, whereas RNA expression was evaluated at prePI, prePIII, 1dPIII, 3dPIII, and 14dPIII (purple cans). The numbers of subjects in the per-protocol cohort (PPC) at study entry and after the outcome of CHMI [either protected (PR), non-protected (NP), or delayed onset of parasitemia (DL)] are indicated (all subjects) and correspond to those subjects who also provided blood samples for IFN-γ measurements. The numbers of subjects from which transcriptome data from microarrays (μA subjects) were derived are indicated below all subjects. The percentages of RTS,S/AS01 recipients over recipients of either vaccine (%AS01) are indicated in parentheses for all subjects and μA subjects after vaccination and after challenge. Note that all 12 non-vaccinated control subjects developed parasitemia within the follow-up period after CHMI (not shown).