Background
The recent West African Ebola epidemic led to accelerated efforts to test Ebola vaccine candidates. As part of the World Health Organisation-led VSV Ebola Consortium (VEBCON), we performed a phase I clinical trial investigating rVSV-ZEBOV (a recombinant vesicular stomatitis virus-vectored Ebola vaccine), which has recently demonstrated protection from Ebola virus disease (EVD) in phase III clinical trials and is currently in advanced stages of licensing. So far, correlates of immune protection are incompletely understood and the role of cell-mediated immune responses has not been comprehensively investigated to date.
Methods: We recruited 30 healthy subjects aged 18–55 into an open-label, dose-escalation phase I trial testing three doses of rVSV-ZEBOV (3 × 105 plaque-forming units (PFU), 3 × 106 PFU, 2 × 107 PFU) (ClinicalTrials.gov; NCT02283099). Main study objectives were safety and immunogenicity, while exploratory objectives included lymphocyte dynamics, cell-mediated immunity and cytokine networks, which were assessed using flow cytometry, ELISpot and LUMINEX assay.
Findings: Immunization with rVSV-ZEBOV was well tolerated without serious vaccine-related adverse events. Ebola virus-specific neutralizing antibodies were induced in nearly all individuals. Additionally, vaccinees, particularly within the highest dose cohort, generated Ebola glycoprotein (GP)-specific T cells and initiated a cascade of signaling molecules following stimulation of peripheral blood mononuclear cells with Ebola GP peptides.
Interpretation: In addition to a benign safety and robust humoral immunogenicity profile, subjects immunized with 2 × 107 PFU elicited higher cellular immune responses and stronger interlocked cytokine networks compared to lower dose groups. To our knowledge these data represent the first detailed cell-mediated immuneprofile of a clinical trial testing rVSV-ZEBOV, which is of particular interest in light of its potential upcoming licensure as the first Ebola vaccine.
VEBCON trial Hamburg, Germany (NCT02283099).
Keywords: rVSV-ZEBOV, Ebola vaccine, Phase I study, T-cell responses, Cytokines, Humoral and cell-mediated immune responses
Highlights
-
•
A phase I clinical trial was conducted to investigate the live-attenuated Ebola vaccine rVSV-ZEBOV.
-
•
Ebola-specific humoral and cell-mediated immune responses show a favorable profile for subjects immunized with 2 × 107 PFU of rVSV-ZEBOV.
-
•
The highest dose cohort induced stronger antigen-specific CTL-responses and interlocked cytokine networks compared to lower dose groups.
rVSV-ZEBOV is the first Ebola vaccine with human efficacy data, currently undergoing an accelerated licensing process. Nevertheless, to date no human immunological correlate of protection has been identified and mechanisms of immune responses elicited by rVSV-ZEBOV remain incompletely understood.
We conducted a phase I trial to test rVSV-ZEBOV in 30 healthy subjects using three dosage levels. We here present a comprehensive evaluation of humoral and cell-mediated responses with an in-depth analysis of signaling molecules following ex vivo stimulation with Ebola GP peptides. Our data suggest a favorable immune response profile for subjects immunized with 2 × 107 PFU.
These data address critical knowledge gaps with respect to mechanisms of immuneprotection in the context of Ebola vaccines and may provide additional evidence to support the current dosage used in later stage clinical trials.
Graphical Abstract
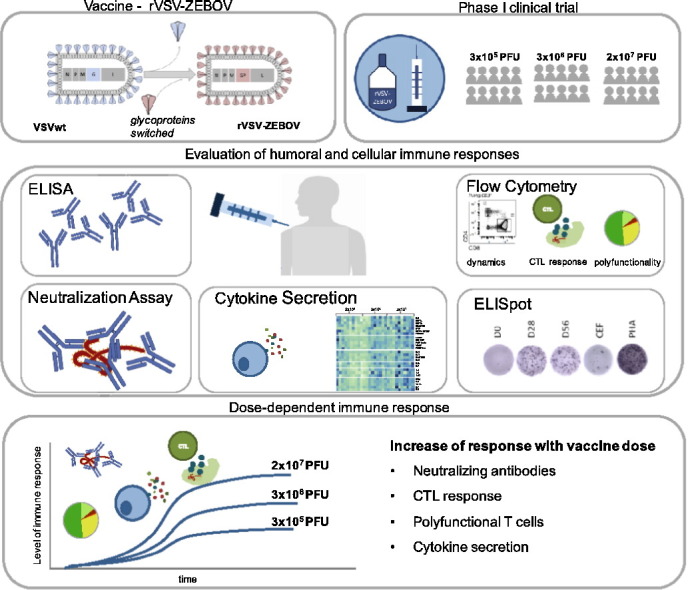
1. Introduction
The recent West African Ebola virus (EBOV) outbreak was the largest outbreak in the history of Ebola virus disease (EVD) with > 28,600 confirmed infections and over 11,300 fatalities (WHO Situation Report, March 2016). This dramatic health crisis was in part facilitated by the lack of licensed medical countermeasures. Following the WHO declaration of the outbreak as a Public Health Emergency of International Concern, clinical studies of Ebola vaccine candidates were accelerated (Pavot, 2016), including rVSV-ZEBOV (recombinant vesicular stomatitis virus-vectored Ebola vaccine).
As part of the WHO-led VSV-Ebola consortium (VEBCON) we conducted a phase I trial to test rVSV-ZEBOV in 30 healthy subjects using three dosage levels (3 × 105, 3 × 106 and 2 × 107 plaque forming units (PFU). The vaccine subsequently proceeded into a phase III trial (Ebola ça suffit!) demonstrating high efficacy in reduction of human-to-human transmission following rVSV-ZEBOV immunization (Henao-Restrepo et al., 2017). The priority licensing process is currently underway with anticipated licensure in 2017. Nevertheless, no human immunological correlate of protection exists and mechanisms of immune responses elicited by rVSV-ZEBOV remain incompletely understood.
rVSV-ZEBOV (serotype Indiana) is genetically modified to encode Ebola glycoprotein (GP) instead of the VSVwildtype GP (Geisbert and Feldmann, 2011). Several animal models revealed protection after lethal challenges with EBOV (Wong et al., 2014, Jones et al., 2005, Marzi et al., 2016, De Wit et al., 2015, Qiu et al., 2009). Non-human primate (NHP) studies demonstrated 100% protection after a single dose of rVSV-ZEBOV (Jones et al., 2005), whereas 50% protection was achieved when it was used as post-exposure prophylaxis (PEP) (Feldmann et al., 2007), suggesting rVSV-ZEBOV as a suitable vaccine candidate during outbreak scenarios.
Mechanisms underlying rVSV-ZEBOV mediated protection are not completely understood thus far. Antibody responses have been correlated with protection in animal models, but could not be demonstrated as surrogate of protection (Marzi et al., 2013, Geisbert et al., 2008, Wong et al., 2012, Jones et al., 2005). The impact of antigen-specific T-cell responses in humans following rVSV-ZEBOV vaccination has not been investigated comprehensively (Pavot, 2016). To date, only one case report, in which rVSV-ZEBOV was used as PEP, reported on lymphocyte dynamics and cellular immunity (Lai et al., 2015). Another study characterized circulating follicular T-helper cells induced by rVSV-ZEBOV vaccination in healthy adults (Farooq et al., 2016). While experimental evidence for T-cell mediated protection from animal models exist (Wilson and Hart, 2001, Wong et al., 2012), more data are needed to understand the contribution of cell-mediated immunity, and specifically Ebola-specific T cells induced by rVSV-ZEBOV for protection against EVD.
Our detailed immune profile revealed increased induction of cell-mediated Ebola-specific immune responses and broader secretion of signaling molecules in subjects immunized with 2 × 107 PFU compared to subjects of the two lower dose groups. Ebola-specific T cells were detectable, albeit at moderate to low magnitude and with CD8+ T-cell predominance in this study. Our data support the dosage of 2 × 107 PFU, as used in the Ebola ça suffit! trial and provide comprehensive novel human information of immune responses elicited by rVSV-ZEBOV.
2. Methods
2.1. Study Design and Participants
NCT02283099 was an open label, phase I investigator initiated trial (IIT) of single-escalating doses of rVSV–ZEBOV (BPSC 1001, also referred to as V920) in healthy adults aged 18 to 55 years. Full details regarding entry criteria and procedures are provided in the study protocol (Supplementary Appendix) and have been described previously (Agnandji et al., 2016). The study was reviewed and approved by the ethics committee, the German authority for genetic engineering, and the WHO research ethics review committee. This study was performed in accordance with the Declaration of Helsinki in its version of Seoul 2008. All participants provided written informed consent. (ClinicalTrials.gov; NCT02283099; Phase I Trial to Assess Safety, Tolerability, and Immunogenicity of Ebola Virus Vaccine).
2.2. Vaccination
The vaccine was developed by New Link Genetics Corporation and the Government of Canada, and manufactured by IDT Biologica GmbH (Dessau, Germany). Injections were administered intramuscularly into the deltoid. Dose-escalation studies were performed in a staggered manner for safety. Participants received doses of 3 × 105, 3 × 106 or 2 × 107 PFU. The vaccination protocol was performed as previously described (Agnandji et al., 2016).
2.3. Safety Monitoring
Local and systemic reactogenicity were recorded for 7 days on a daily notification sheet after vaccination and were reported further on follow-up visits. Safety monitoring was performed as previously described (Agnandji et al., 2016).
2.4. Immunogenicity
Sera were collected to perform enzyme-linked immunosorbent assay (ELISA) for EBOV GP-specific antibodies using inactivated whole virions of the Zaire-Guéckédou strain. Neutralizing antibodies were detected using VSV-pseudovirions expressing the luciferase reporter gene, or by using infectious EBOV-isolate (Mayinga). The latter one was done with sera starting with a dilution of 1:8. Seropositivity is defined by a GMT < 8. The assays were performed as previously reported (Agnandji et al., 2016, Krahling et al., 2016).
2.5. T- and B-cell Phenotype and Dynamics
Peripheral blood mononuclear cells (PBMCs) were isolated and cryopreserved from EDTA-blood using standard operating procedures via ficoll density gradient centrifugation. Phenotypic properties of T and B cells were analyzed by flow cytometry using LSRFortessa (BD Bioscience) and evaluated with FlowJo10. Flow cytometry gating strategy and the corresponding used antibodies are shown in the Supplementary Appendix.
2.6. Ebola-specific T-cell Responses
Antigen-specific T cells were analyzed using cryopreserved PBMCs. Following overnight resting, PBMCs were incubated for 6 h at 37 °C with overlapping peptide pools (OLPs) spanning the aa sequence of Ebola GP (Kikwit-strain, sequences: Supplementary Appendix: Peptide pool) in the presence of CD28/CD49d, GolgiStop and GolgiPlug. Negative controls were treated with RPMI1640 (10% FCS supplemented with DMSO). PMA and CEF (CMV,EBV,Influenza-peptides) served as positive controls. We used IC fixation and Perm buffer (affymetrix ebioscience) to analyze the expression of tumor necrosis factor α (TNFα), interleukin 2 (IL2), macrophage inflammatory protein 1β (MIP1β) and interferon γ (IFNγ); and stained also against CD107a. Cells were analyzed on LSRFortessa (BD Bioscience) and evaluated with FlowJo10. Gating strategy and corresponding antibody panels are depicted in the Supplementary Appendix S1 and Table S3. Polyfunctionality was analyzed using Boolean gating. The measured values were subtracted for each sample with the corresponding DMSO control.
2.7. Measurement of Cytokines, Chemokines and Growth Factors
Ebola GP-specific cellular responses were assessed using Ebola GP OLPs by IFNγ-ELISpot (MabtechELISpotPLUS). Cryopreserved PBMCs were rested for 4 h followed by 16 h stimulation with peptide pools. We used 125,000 cells/well and performed the ELISpot as previously described (Dahlke et al., 2017). PHA and CD3 were used as positive controls, RPMI1640 (10%FCS supplemented with DMSO) served as negative control.
Cytokines, chemokines and growth factors were measured using supernatant of the ELISpot assay, which was collected 16 h after stimulation. Samples were analyzed using a 27plex-BioRad-LUMINEX assay following the manufacturers instructions. Variables were ordered by hierarchical clustering with hclust provided with complete as agglomeration method. R (3.3.2) (https://www.R-project.org/) was applied for calculation of the correlations and plotting. Correlations were plotted using package corrplot (0.77) (https://CRAN.R-project.org/package=corrplot), the heatmap was plotted using package pheatmap (1.0.8) (https://CRAN.R-project.org/package=pheatmap).
2.8. Data Analysis
Frequencies of adverse events (AEs) and serious adverse events (SAEs) were determined per dose group. We employed Fisher's exact test, Mann-Whitney-Wilcoxon, Kruskal-Wallis test to calculate intra- and inter-group associations as appropriate. Analyses were performed in Prism (v7.0a, GraphPad Software, San Diego, CA).
3. Results
3.1. Study Participants
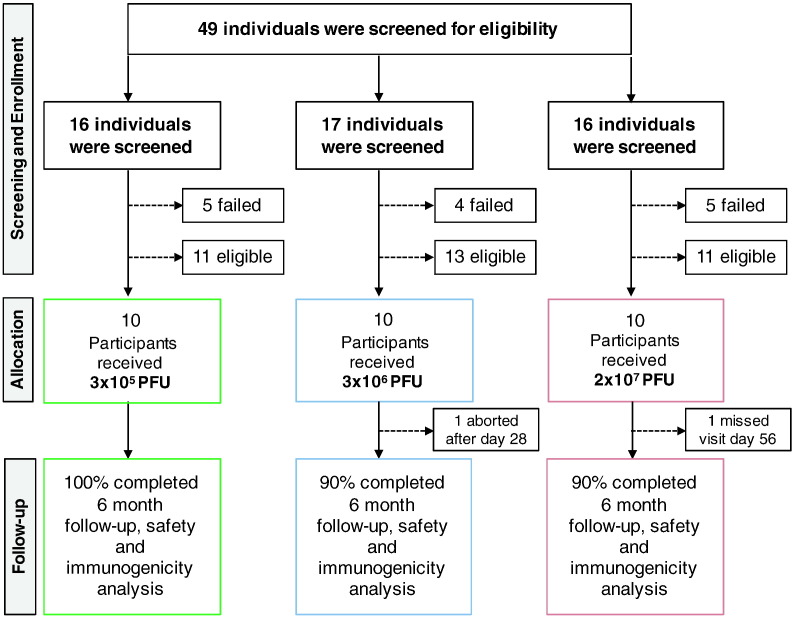
A total of 30 participants were vaccinated at the University Medical Center Hamburg Eppendorf (UKE) between November 17, 2014 and May 5, 2015. The study population consisted of 20 men and 10 women with a mean age of 38.8 years (range: 23–54 years) (Table 1). 29 participants were of European, one was of Asian descent. The follow-up visits were attended by all participants except one, who exited the study after day 28 (Fig. 1). Clinical data of part of the study population were reported previously (Agnandji et al., 2016). We here present the complete data set of the Hamburg VEBCON study site.
Table 1.
Patient characteristics at baseline. Characteristics of individuals at baseline.
| Cohort 1 3 × 105 PFU |
Cohort 2 3 × 106 PFU |
Cohort 3 2 × 107 PFU |
|
|---|---|---|---|
| Sex — no. (%) | |||
| Male | 7 (70) | 6 (60) | 7 (70) |
| Female | 3 (30) | 4 (40) | 3 (30) |
| Age — years | |||
| Mean | 44 ± 11 | 32 ± 8 | 40 ± 9 |
| Range | 23–54 | 24–47 | 24–51 |
| Race — no. (%) | |||
| Asian | 0 | 1 (10) | 0 |
| White | 10 (100) | 9 (90) | 10 (100) |
| Body-mass index | 27 ± 3 | 25 ± 3 | 24 ± 3 |
Plus-minus values are means ± SD.
Race was self-reported.
The Body-mass index is the weight in kilograms divided by the square of the height in meters.
Fig. 1.
Trial profile. Flow diagram of screening, enrollment and vaccination in the three cohort groups. For each cohort group, 4 to 5 eligible individuals withdrew from the study. One participant aborted the study after day 28 due to moving abroad (3 × 106 PFU). One subject missed visit day 56 (2 × 107 PFU).
3.2. rVSV-ZEBOV Exhibits a Benign Safety Profile
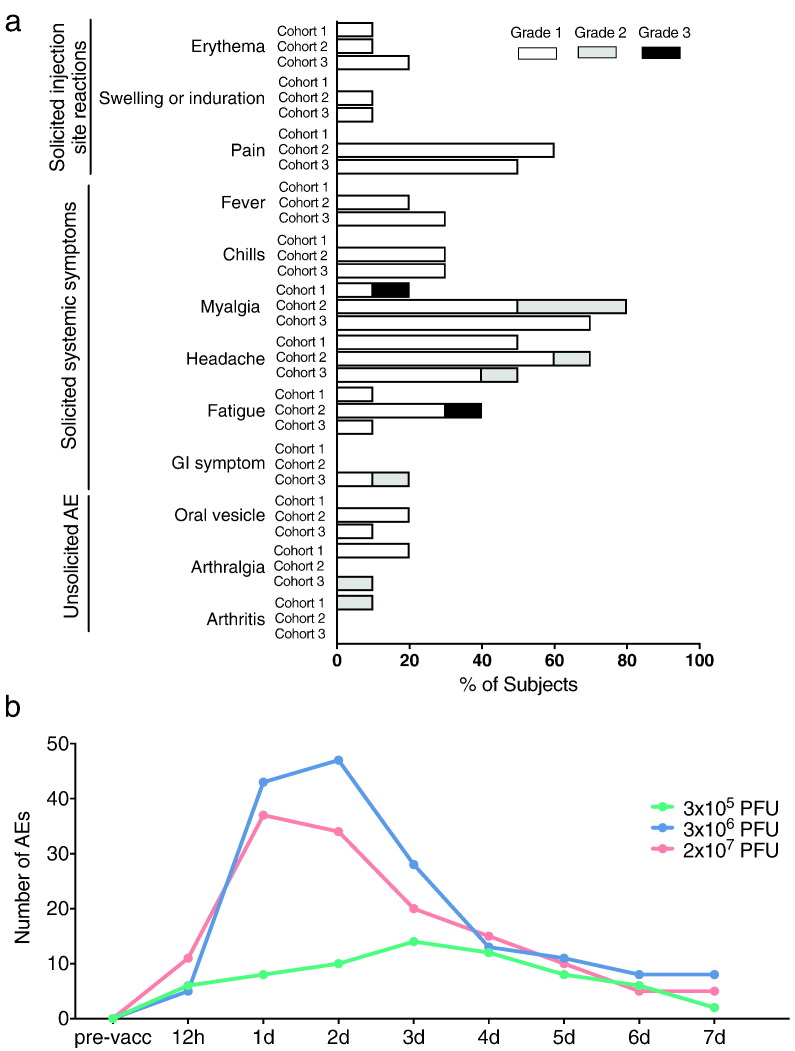
In the final analysis of the complete Hamburg cohort, no vaccine-related SAEs or AEs resulting in withdrawal from the study were observed. During the six month follow-up, all participants (except one) experienced AEs. The majority of AEs (104) were classified as mild, 20 as moderate and three as severe (neck-tension, myalgia and fatigue) (Fig. 2a, neck tension is excluded from Fig. 2a as it was a single event). All of them were transient (mean duration was 2.14 days, 95% CI 1.80–2.48). The most frequent AEs were pain at the injection site, myalgia and headache. Solicited local reactogenicity was common but generally mild. Fever, but also viremia and lymphopenia had been observed in the two higher dose groups, but not in subjects immunized with the lowest dose (Supplementary Appendix, Figs. S2, S3 and Tables S5, S6).
Fig. 2.
Local and systemic adverse events. a) Recorded adverse events of vaccinees during 180 days post vaccination. Events are depicted in frequency (%) of participants among respective cohort presenting at least one adverse event of the category. Adverse events with the severity of mild, moderate and severe are grouped into grade 1, 2 and 3, respectively. Cohort 1: 3 × 105 PFU, Cohort 2: 3 × 106 PFU, Cohort 3: 2 × 107 PFU. b) Number of related solicited and unsolicited adverse events reported over time in the 3 × 105 PFU (cohort 1 (green)), 3 × 106 PFU (cohort 2 (blue)), and 2 × 107 PFU (cohort 3 (red)) dose group.
The majority of AEs occurred within the first week post vaccination (Fig. 2b). Subjects of the two higher dose cohorts reported a higher number of AEs during the first four days with a peak at day 1 and 2 post vaccination compared to the lowest dose group, which overall experienced a lower number of AEs with a peak on day 3.
Four vaccinees experienced unsolicited AEs; three cases of arthralgia and one case of oligoarthritis were reported. They were transient and of mild to moderate intensity. The case of oligoarthritis was observed in the lowest dose group and had not been reported in the previous report (Agnandji et al., 2016).
3.3. rVSV-ZEBOV Induces Neutralizing Antibody Responses
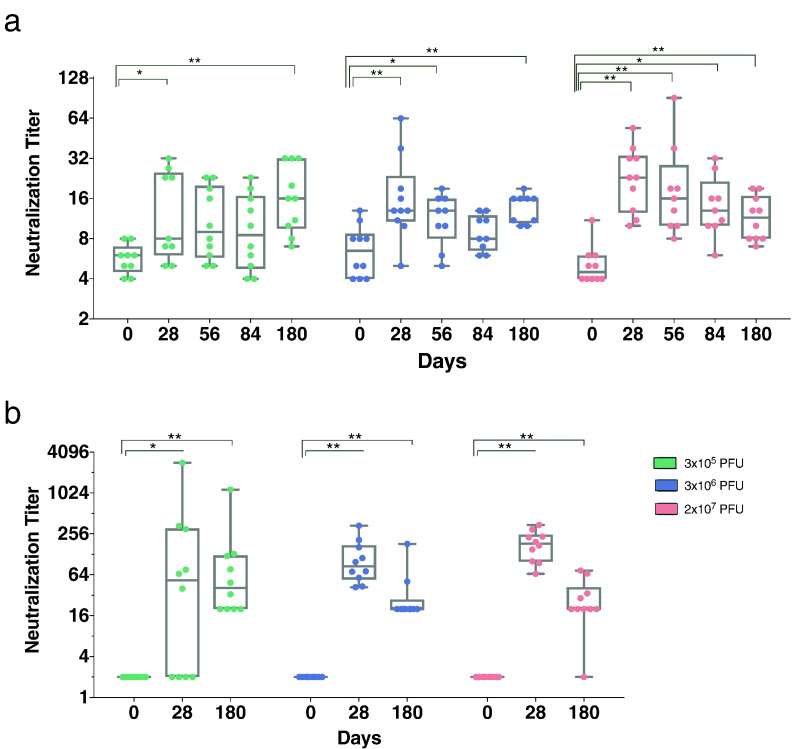
Ebola GP-specific IgG responses were assessed using three different assays (Fig. 3 and Supplementary Appendix, Fig. S4). The ELISA with whole inactivated Ebola virions revealed an early induction at day 14 in the highest dose cohort that persisted for six months (Fig. S4a).
Fig. 3.
Ebola virus antibody titers increase following administration of rVSV-ZEBOV. a) Neutralizing antibodies against infectious EBOV isolate Mayinga were analyzed. Analysis was started with a 1:8 dilution. Seropositivity is defined by a GMT > 8. b) Pseudovirion neutralization assay assessing the 50% serum neutralization capacity (PsVNA50) complemented by homologous glycoprotein. a) and b): The results are expressed in neutralization titers. Statistical analysis was performed using Mann-Whitney-Wilcoxon test (*p < 0.05; **p < 0.01; ***p < 0.005) (Graphs: Box and Whiskers, Min to Max, Line: Median).
Titers of neutralizing antibodies were determined using infectious EBOV-isolate (Fig. 3a). While all subjects (100%) immunized with 2 × 107 PFU revealed a > 2-fold induction to baseline, 3 × 105 and 3 × 106 PFU cohorts showed > 2-fold induction in 67% and in 60% of the subjects, respectively (Fig. 3b, fold inductions are depicted in Supplementary Appendix, Table S7). The pseudovirion neutralization assay was performed assessing 50% serum neutralization at day 0, 28 and 180 post vaccination. (Fig. 3c). This assay showed an increase of neutralizing antibodies on day 28 that persisted until day 180, with the notable exeption of one subject, in which a decrease to baseline was observed. In summary, both assays showed a significant induction of neutralizing antibodies after any dose of rVSV-ZEBOV, but revealed no intergroup differences using the Kruskal-Wallis test (Supplementary Appendix, Fig. S4).
3.4. Dynamics of Lymphocyte Responses
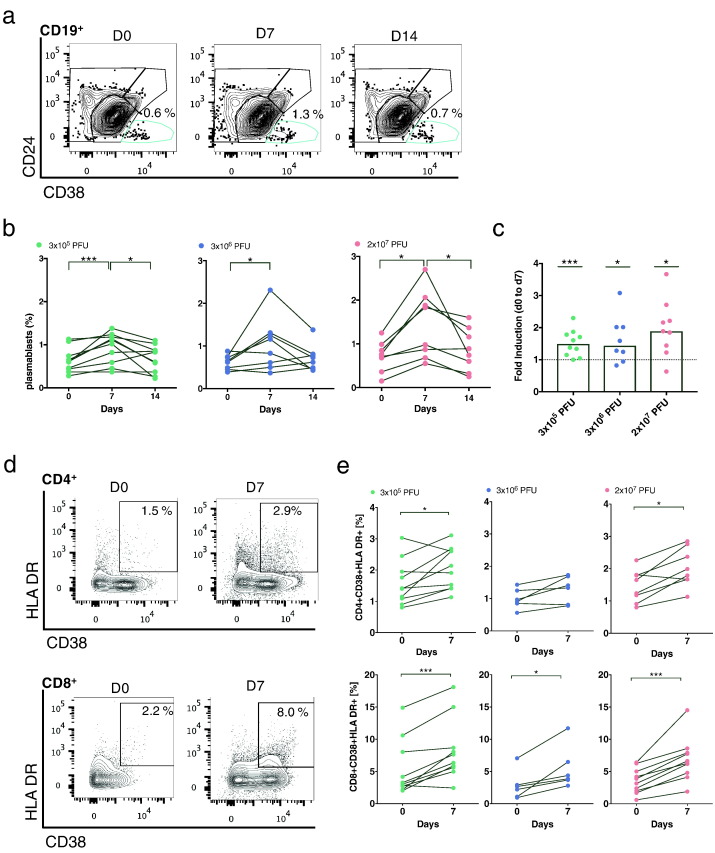
To date, no flow cytometry data on lymphocyte dynamics and activation following vaccination in humans have been reported for rVSV-ZEBOV vaccine trials. We therefore first investigated B-cell dynamics via staining CD19, CD24 and CD38 (Fig. 4a) and observed a peak in the number of plasmablasts (CD19+ CD24− CD38+) at day 7 (Fig. 4b). While this trend was significant in all groups (Fig. 4c), 44% of the 2 × 107 PFU cohort showed a > 2-fold induction compared to 37.5% and 10% of vaccinees of the 3 × 106 and 3 × 105 PFU dose cohort, respectively. Nevertheless, we identified no intergroup differences (Kruskal-Wallis test).
Fig. 4.
Kinetics of plasmablasts and T-cell activation in response to rVSV-ZEBOV immunization. a) Plasmablasts peaked at day 7 post vaccination in all dose groups. B cells were stained using antibodies against CD19, CD24 and CD38. Representative contour plots depict B-cell subsets of naïve (CD19+ CD24low/intermCD38interm), memory (CD19+ CD24−/interm/highCD38low/interm), transitional (CD19+ CD24+ CD38high) and plasmablasts (CD19+ CD24− CD38high). Numbers in contour plots represent the percentage of plasmablasts of CD19+ B cells. b) Percentages of CD19+ CD24− CD38high over time in immunized subjects. Each dot and line depicts one subject (3 × 105 PFU: n = 10; 3 × 106 PFU: n = 8; 2 × 107 PFU: n = 9). c) Graph highlights the fold induction of plasmablasts at day 7 compared to day 0. Each dot represents one subject (bar graph, line: median value). d) T-cell activation in subjects early after vaccination. Activation was analyzed by HLA DR and CD38 staining. Representative contour plots depict the gating strategy for activation of CD4+ (upper plot) and CD8+ T cells (lower plot). e) Percentages of HLA DR+ CD38+ of CD4+ (upper row) and CD8+ T cells (lower row) are represented in the graphs. Each dot and line represents one subject. (3 × 105 PFU: n = 10; 3 × 106 PFU: n = 6; 2 × 107 PFU: n = 8). Statistical analysis was performed using Mann-Whitney-Wilcoxon test (*p < 0.05; **p < 0.01; ***p < 0.005).
Next, we assessed the activation status of both CD4+ and CD8+ T cells by staining with anti-CD38 and anti-HLA DR antibodies (Fig. 4d). Both types of T-cell subsets showed an increased activation status at day 7 compared to baseline (Fig. 4e), with higher activation levels in the CD8+ T-cell compartment compared to the CD4+ T cells. All dose groups exhibited a significant induction of activated CD8+ T cells at day 7 (Mann-Whitney-Wilcoxon test: 3 × 105: p value 0.004; 3 × 106: p value 0.03; 2 × 107: p value 0.002).
3.5. rVSV-ZEBOV Induces Detectable Ebola GP-specific T-cell Responses
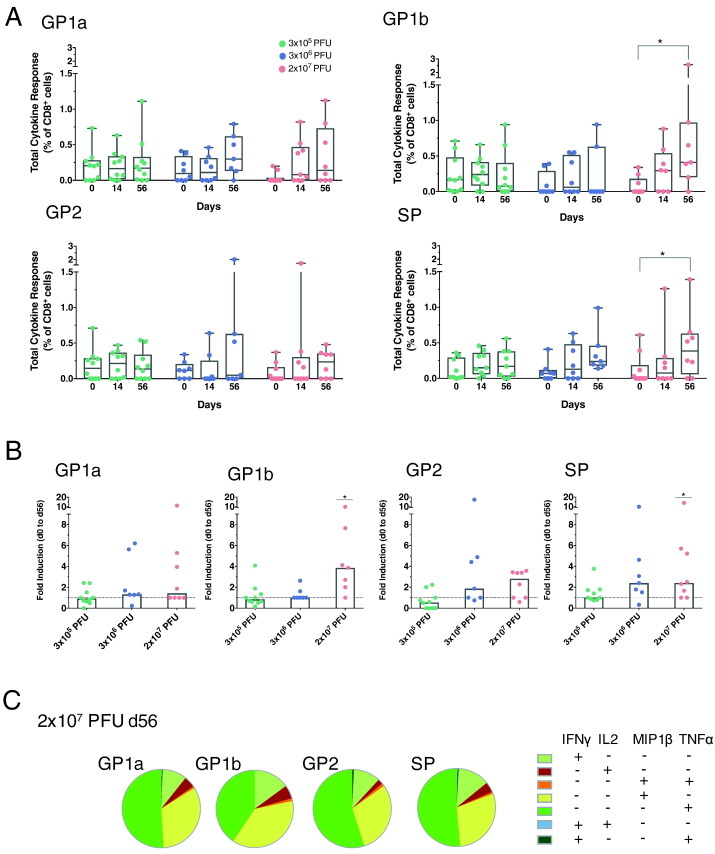
To investigate the presence of Ebola GP-specific cell-mediated responses following vaccination, we assessed the induction of cytokines (TNFα, IFNγ, IL2, MIP1β) after stimulation with four OLP-pools that cover Ebola GP (GP1a, GP1b, GP2 and SP) (Fig. 5a). Overall T-cell responses were low to moderate and predominantly observed in the CD8+ T-cell compartment of the highest dose cohort upon stimulation with the peptide pools GP1b and SP (GP1b: p value 0.03; SP: p value 0.03) (results from CD4+ cells are shown in the Supplementary Appendix, Fig. S6). Induction of cytokine responses were predominantly increased (> 2 fold) from day 0 to 56 in the highest dose cohort after stimulation, with strongest responses following GP1b stimulation. A > 2 fold induction was obsered in 85% of subjects of the highest dose cohort, while the lower dose cohorts showed this response in 14% and 10% of subjects (3 × 106 PFU, 3 × 105 PFU, respectively). We detected no intergroup variations (Supplementary Appendix, Figs. S5 and S6).
Fig. 5.
Ebola GP-specific T-cell responses. Kinetics of antigen-specific CD8+ T-cell responses. a) Four different peptide pools (GP1a, GP1b, GP2, SP) that cover the whole Ebola GP protein (Kikwit) were used for PBMC stimulation. Graphs represent frequencies (%) of total cytokine responses of CD8+ T cells (IFNγ, TNFα, IL2, MIP1β). Each dot represents one subject. (3 × 105 PFU: n = 10; 3 × 106 PFU: n = 7; 2 × 107 PFU: n = 8) (Box and Whiskers, Min to Max, Line: median) b) Fold induction of total cytokines of CD8+ T cells from day 0 to day 56 (Bar graph, line: median value). c) Piecharts represent composition of cytokines induced by all four peptide pools. They show the average values of CD8+ T-cell responses of day 56 of the 2 × 107 PFU dose cohort.
We could not detect a significant expansion of polyfunctional CD8+ T cells, as representatively depicted in the piecharts for the highest dose cohort against the GP1b pool (day 56) (Fig. 5c). The largest fraction of GP1b-specific T cells were CD8+ T cells secreting either IFNγ, TNFα or MIP1β. The relatively low level of IFNγ secretion was validated by an IFNγ-ELISpot, which is depicted in the Supplementary Appendix Fig. S8.
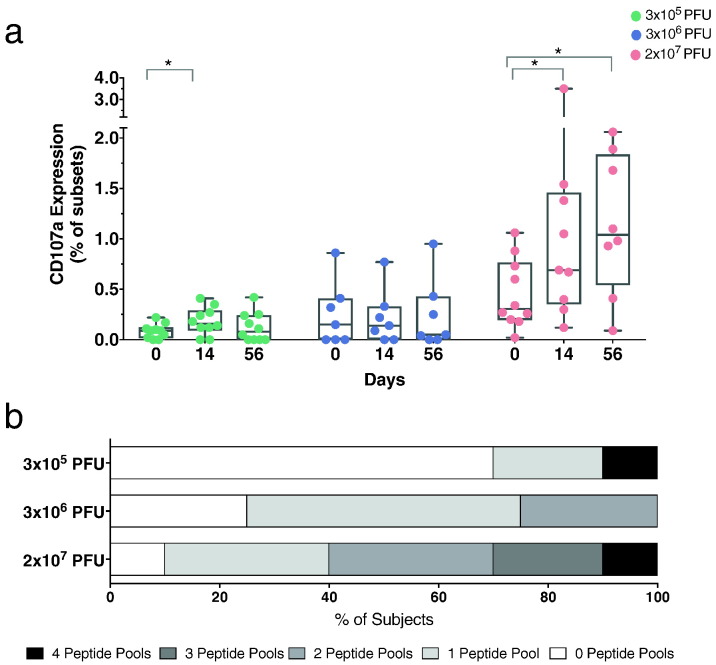
In addition to cytokine expression, we investigated CTL-responses using a CD107a degranulation assay. In line with the cytokine responses, expression of CD107a by CD8+ T cells was increased at day 56 post vaccination in the highest dose cohort following stimulation with Ebola GP OLP-pools (Fig. 6a). Intergroup differences were identified at day 14 and 56 post vaccination (Supplementary Appendix, Fig. S7). To analyze the breadth of detected CTL-responses, we examined the number of pools per subject that showed positive responses. Positivity was specified as three times over the baseline. The breadth of the CTL-response was greatest in subjects immunized with the highest dose (Fig. 6b).
Fig. 6.
CTL-responses following rVSV-ZEBOV vaccination. a) Degranulation of CD8+ T cells was measured by CD107a staining. Graphs depict the sum of frequency of CD107a expression induced by all four peptide pools (GP1a, GP1b, GP2, SP) (Box and Whiskers, Min to Max, Line: median). b) The breadth of peptide pool responses increases by vaccine dose. An assay response was determined by 3-fold over the background. The darker the grey, the more peptide pool responses, the higher the breadth of response. Statistical analysis was performed using Mann-Whitney-Wilcoxon test (*p < 0.05; **p < 0.01; ***p < 0.005).
3.6. Vaccine Dose Associates With Cytokine Secretion (Supernatant)
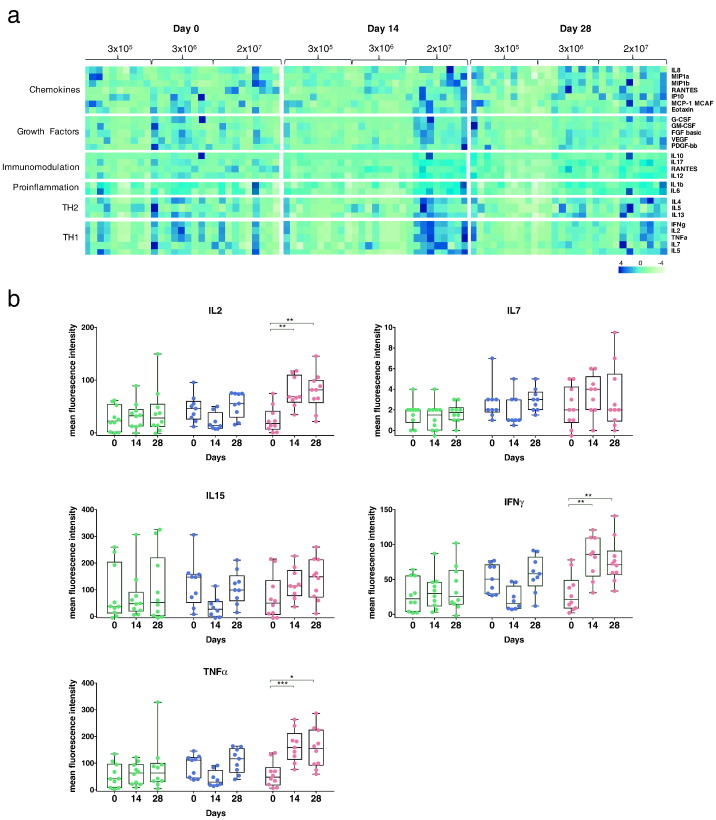
For the identification of rVSV-ZEBOV induced cytokine, chemokine and growth factor patterns, supernatant from Ebola GP OLP-stimulated PBMCs were analyzed using a 27-plex-LUMINEX assay. This approach allows for in-depth analysis of signaling molecules belonging to six functional classes that mediate the immune response (chemokines, growth factors, TH2, TH1, immunomodulation, pro-inflammation) (Fig. 7a). The highest dose group induced a broad secretion profile at day 14, while the medium dose cohort showed a delayed increase at day 28. Cytokines belonging to the TH1-response (IL2, IL7, IL15, IFNγ, TNFα) revealed strong and early induction in subjects immunized with 2 × 107 PFU, which is highlighted in Fig. 7b. Notably, specifically IL2, IFNγ and TNFα revealed a significant intergroup difference between all dose groups at day 14 and 28 post vaccination (Kruskal-Wallis test; see also Supplementary Appendix, Fig. S10). Strong intergroup differences were also observed for IP10 (Supplementary Appendix, Figs. S9 and S10) showing as well significant intra- and intergroup differences. IP10 is one of the most potent chemokine that induces inflammatory responses and is discussed as a factor for immunologic enhancement (Kang et al., 2009, Nakaya et al., 2011).
Fig. 7.
PBMCs induce broad cytokine profile following stimulation with Ebola GP peptide pools. a) PBMCs were stimulated with two peptide pools (MP1, MP2 (Appendix Table 5)) that cover the whole Ebola GP (Kikwit). Following 16 h stimulation, supernatants of both stimulations were pooled together and a 27-plex LUMINEX assay was performed (blue: high expression, green: low expression). b) TH1-belonging cytokines are induced in subjects immunized with 2 × 107 PFU. Graphs depict the cytokines IL2 (upper left graph), IL7 (upper right graph), IL15 (middle left graph), IFNγ (middle right graph) and TNFα (lower left graph) (Box and Whiskers, Min to Max, Line: median). Statistical analysis was performed using Mann-Whitney-Wilcoxon test (*p < 0.05; **p < 0.01; ***p < 0.005).
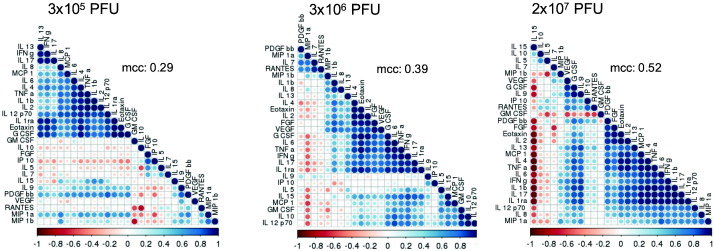
Since cytokines form a coordinating network mediating immune response, we further investigated specific correlations using correlogram analysis (Fig. 8). The strongest interconnections between cytokines at day 14 post vaccination were observed in the highest dose cohort as indicated by the median correlation coefficient (mcc) (2 × 107 PFU: mcc 0.52; 3 × 106 PFU: mcc 0.39; 3 × 105 PFU: mcc 0.29). While IFNγ and TNFα were grouped into two separate clusters in the lowest dose group, the increase of the vaccine dose established a stronger correlation of these two cytokines as summarized in Fig. 8.
Fig. 8.
Cytokines show an interlocked network following peptide stimulation. Correlogram of correlations among 27 cytokines for all dose cohorts for day 14 following vaccination. The highest median correlation coefficient (mcc) was observed in the 2 × 107 PFU cohort with 0.52 in contrast to 0.39 and 0.29 in the middle and lowest dose group, respectively. Red indicates a negative and blue a positive correlation. The more intense the colour, the greater the correlation magnitude. Correlogram was plotted using R software.
4. Discussion
In the aftermath of the recent EVD outbreak, significant progress has been made in Ebola vaccine development. rVSV-ZEBOV currently represents the most promising vaccine candidate, specifically following recent results from the Ebola ça suffit! trial suggesting a high level of efficacy (Henao-Restrepo et al., 2017). While these results and the pending licensure of rVSV-ZEBOV are encouraging, the exact correlates of immune-protection remain unknown and human immunity data on this novel vaccine vector system are scarce.
In addition to safety and humoral responses for the complete Hamburg study population, to our knowledge we here report the first comprehensive analysis of B- and T-cell responses, including Ebola-specific T-cell responses following rVSV-ZEBOV immunization. The present study provides a detailed account of lymphocyte dynamics, breadth and magnitude of Ebola GP-specific T-cell responses and cytokine patterns. The results support the use of the vaccine dose 2 × 107 PFU in healthy adults, which corresponds to the dose administered in the recent phase III clinical trial in Guinea (Henao-Restrepo et al., 2017) and currently prepared for licensing.
As in previous studies reported by us and others, the vaccine was found to be reactogenic, but safe and well-tolerated without vaccine-related SAEs (Regules et al., 2017, Agnandji et al., 2016). Not unexpectedly, the higher dose cohorts (3 × 106, 2 × 107) experienced increased reactogenicity peaking at days 1–2 post vaccination, however no significant dose-effect was detected (Fig. 2b, Kruskal-Wallis test, 0.5 p value).
Based on this current and our previously reported data, a single immunization of rVSV-ZEBOV significantly induced antibody responses in humans, which were maintained for at least 6 months independent of dosage level. The impact of antibodies to protect against EVD following rVSV-ZEBOV immunization was demonstrated in a study, where rVSV-ZEBOV immunized NHP succumbed to lethal EBOV-challenge after loss of Ebola GP-specific antibodies following CD4+ T-cell depletion during vaccination (Marzi et al., 2013). However, binding or neutralizing antibodies as sole immune correlates could not be confirmed so far (Sullivan et al., 2009). In this context, a higher proportion of rVSV-ZEBOV-induced IgM antibodies in contrast to IgG were recently described suggesting a potential over-estimation of the impact of vaccine-induced IgG antibodies (Khurana et al., 2016).
Data from pre-clinical studies indicated involvement of humoral and cellular mechanisms in vaccine-mediated immune protection (Marzi et al., 2013, Jones et al., 2007, Sullivan et al., 2003, Sullivan et al., 2009). However, the role of cell-mediated responses induced by rVSV-ZEBOV, but also in natural EBOV-infection has scarcely been analyzed. Some limited T-cell data from our group and others exist that characterize Ebola GP-specific T cells from EVD-survivors, rVSV-ZEBOV immunized NHP, a rVSV-ZEBOV-PEP case as well as from individuals immunized by adenovirus-vectored Ebola vaccines (Jones et al., 2005, Dahlke et al., 2017, Ruibal et al., 2016, Mcelroy et al., 2015, Marzi et al., 2015, De Santis et al., 2016, Qiu et al., 2009). All studies commonly identified a predominance of EBOV GP-specific CD8+ over CD4+ T cells that express IL2, IFNγ and/or TNFα (Jones et al., 2005, Mcelroy et al., 2015, Marzi et al., 2015, De Santis et al., 2016, Qiu et al., 2009). Our study revealed detectable Ebola GP-specific T cells with a low proportion of polyfunctional responses, which is in line with findings observed in rVSV-ZEBOV-immunized NHP and EVD-survivors (Mcelroy et al., 2015, Dahlke et al., 2017), but also for the vaccine HPV3/EboGP (Meyer et al., 2015).
While IFNγ induction has been shown as an immune correlate for some diseases, it might play a minor role in EVD-protection. Animal studies revealed that cytolytic activity may correlate better with EVD-clearance than IFNγ secrection (Meyer et al., 2015, Gupta et al., 2005, Wilson and Hart, 2001). In line with this, we also found little Ebola-specific IFNγ secretion but dose-dependent and significant induction of antigen-specific CTL-responses as measured by CD107a. However, for lack of data evaluating the capacity of antigen-specific T cells to protect against EBOV-challenge, it remains unknown or equivocal whether these cells contribute significantly to protection. The question, to what extent Ebola-specific T cells of low-to-moderate magnitude contribute to vaccine-mediated immune protection remains unanswered and warrants further study. Of note, we observed a general activation of T cells in the vaccinees, which seems to be non-Ebola specific and might very well be a hallmark of immunization with replicating vaccines as a similar phenomenon has been observed for YFV-17D (Blom et al., 2013, Miller et al., 2008).
While flow cytometry- and ELISpot-based investigation allows for the assessment of only a limited number of cytokines, multiplexed LUMINEX-analysis supports in-depth inquiry of a broad array of rVSV-ZEBOV induced signaling molecules. We observed a substantial secretion upon stimulation with Ebola GP OLP-pools. In particular, cytokines belonging to TH1-response revealed a significant induction in subjects immunized with 2 × 107 PFU. The cytokines IL2, IFNγ and TNFα, which activate antigen-presenting cells, growth and survival of antigen-specific cytotoxic cells, were significantly induced. Of note, the highest dose cohort also significantly induced IP10 (Supplementary Appendix, Figs. S9 and S10), which, based on our unpublished data, might also serve as a predictive marker for GP-specific antibody titers (unpublished data; Rechtien et al.).
In addition to cytokine induction, our correlogram analysis data revealed that different vaccine doses generate a new order within the cytokine network. The complexity and interconnectivity associated with the vaccine dose, as we observed strongest correlations between signaling molecules in the 2 × 107 PFU cohort. This is concordant with and extends data by Farooq et al., in which 10 cytokines were analyzed in rVSV-ZEBOV immunized subjects (Farooq et al., 2016). While direct comparison is limited by the different numbers of cytokines analyzed (27 in the present study versus 10 cytokines), both studies revealed that the interlocked network increases with vaccine dose. Farooq and colleagues did not detect a correlation of TNFα and IFNγ as we have observed, but both studies identified a cluster consisting of IL4, IL6, IL1b and TNFα. Although neither of these molecules nor their correlation have so far been associated with EVD protection, it can be speculated that they potentially play a role in and contribute to protection against the disease.
As the licensing process of the vaccine is currently underway, the aspect of variable responses to rVSV-ZEBOV in individuals with different genetic background, especially in individuals of African descent, requires attention. Our study exclusively included participants of European descent except one of Asian descent. Consequently, the results presented here cannot address this issue. However, our previous study, in which parts of our clinical data were reported, revealed only minor differences in antibody titers from subjects with African and European descent (Agnandji et al., 2016). While this raising the possibility that similar effects may hold true for cellular responses, this question warrants further study.
In summary, we here report a side-by-side description of humoral and cell-mediated responses, including Ebola-specific T cells following immunization with the first Ebola vaccine candidate demonstrating efficacy in humans. We delineate a comprehensive human immune profile of rVSV-ZEBOV, for which very limited human data exist, especially as it also represents a new vaccine vector platform in human use. While no strong dose-dependence was identified with respect to safety, lymphocyte dynamics and binding antibodies, our data support the usage of 2 × 107 PFU based on EBOV GP-specific T-cell responses and cytokine profiles, underscoring our previous data suggesting higher neutralizing antibody titers in the 2 × 107 PFU dose cohorts (Agnandji et al., 2016). Since the exact roles of neutralizing antibodies, cell-mediated responses and cytokines in EVD-protection remain unknown, it is difficult to conclude from these whether higher vaccine doses are required for optimal protection.
In conclusion, our results provide critical new insight into cellular immunity following rVSV-ZEBOV vaccination and contribute to our understanding of immunity elicited by this novel vaccine vector platform in humans. While antibodies usually protect against infection, cytokines as well as CD8+ T cells might be critical factors to terminate virus-replication and thereby control viremia and infection. These data contribute importantly to our understanding of EVD-immunity and may lay a foundation for further strategic vaccine design in the context of next generation emergency vaccines.
Funding Sources
This work was supported in part by research funding from the Wellcome Trust, (SPHQ14-LOA-311) the German Center for Infection Research (TTU01.905) (EBOKON; DZIF with the partner sites Tübingen, Gießen-Marburg-Langen and Hamburg-Lübeck-Borstel), the German National Department for Education and Research (BMBF) and the Bundesministerium für Gesundheit (BMG) (ZMVI5-2514NIK005).
Conflicts of Interest
We declare no conflicts of interests.
Author Contributions
MMA is the principal investigator of this study with SS and AJ as deputies with overall oversight of study, design, analysis and write-up. AWL and StB made substantial contributions to the conception and design of the study. SB supervised the process of the study at the study site, including study design, recruitment, follow-up. AJ organized the safety assessment. FS took responsibility for safety laboratory analyses. MZ, CD, RK supervised the process of the laboratory site. They were supported by AR, HCS and MLL. T-cell assays and Luminex assays were designed, performed and evaluated by CD, RK and SL. MLL supported the performance of all T-cell assays. VK, SKF, TS, ME performed the assays to analyze antibody binding titers and neutralizing antibodies. CD and RK performed detections of viral RNA via qRT PCR, which was supported by ML. MS generated the correlogram and heatmap. FO evaluated statistically the data sets. CD and MMA wrote the report, which was critically reviewed by RK, SL, MZ and FO.
Acknowledgements
We thank all volunteers for their participation in this first-in-human phase I vaccine trial and their commitment to combat the recent and further outbreaks of EVD, and the Public Health Agency of Canada for donating the vaccine to the WHO. We would also like to express our sincere gratitude to all trial center members for their extraordinary work (Clinical Trial Center North GmbH & Co. KG, Hamburg). We are grateful for laboratory support by Prof. Thomas Renné and team and all laboratory members of the University Medical Center Hamburg-Eppendorf (UKE); and thank Jay Hooper and Peter Silver (USAMRIID) for performing the pseudovirion neutralization assay, which data has been previously shown by Agnadji et al.
Furthermore, we thank the Paul-Ehrlich-Institute as well as the respective national competent authorities and local ethics committees and authorities for genetic engineering as well as WHO Research Ethics Review Committee for their exceptionally rapid reviews. We are indebted to the DSMB members Prof. Markus Müller, Prof. Bernhards Ogutu, Prof. Tim Peto, Prof. Jürgen May and Prof. Klara Posfay-Barbe for their commitment and oversight. We also thank the Hamburg local safety board members: in particular Prof. Jan Rupp.
Footnotes
Funding: Wellcome Trust through WHO (SPHQ14-LOA-311), BMG (ZMVI5-2514NIK005), BMBF and DZIF (TTU01.905).
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2017.03.045.
Contributor Information
Marylyn M. Addo, Email: m.addo@uke.de.
VEBCON Consortium:
Marylyn M. Addo, Stephan Becker, Verena Krähling, Selidji Todagbe Agnandji, Sanjeev Krishna, Peter G. Kremsner, Jessica S. Brosnahan, Philip Bejon, Patricia Njuguna, Claire-Anne Siegrist, Angela Huttner, Marie-Paule Kieny, Kayvon Modjarrad, Vasee Moorthy, Patricia Fast, Barbara Savarese, and Olivier Lapujade
Appendix A. Supplementary data
1
2
References
- Agnandji S.T., Huttner A., Zinser M.E., Njuguna P., Dahlke C., Fernandes J.F., Yerly S., Dayer J.A., Kraehling V., Kasonta R., Adegnika A.A., Altfeld M., Auderset F., Bache E.B., Biedenkopf N., Borregaard S., Brosnahan J.S., Burrow R., Combescure C., Desmeules J., Eickmann M., Fehling S.K., Finckh A., Goncalves A.R., Grobusch M.P., Hooper J., Jambrecina A., Kabwende A.L., Kaya G., Kimani D., Lell B., Lemaitre B., Lohse A.W., Massinga-Loembe M., Matthey A., Mordmuller B., Nolting A., Ogwang C., Ramharter M., Schmidt-Chanasit J., Schmiedel S., Silvera P., Stahl F.R., Staines H.M., Strecker T., Stubbe H.C., Tsofa B., Zaki S., Fast P., Moorthy V., Kaiser L., Krishna S., Becker S., Kieny M.P., Bejon P., Kremsner P.G., Addo M.M., Siegrist C.A. Phase 1 trials of rVSV Ebola vaccine in Africa and Europe. N. Engl. J. Med. 2016;374:1647–1660. doi: 10.1056/NEJMoa1502924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blom K., Braun M., Ivarsson M.A., Gonzalez V.D., Falconer K., Moll M., Ljunggren H.G., Michaelsson J., Sandberg J.K. Temporal dynamics of the primary human T cell response to yellow fever virus 17D as it matures from an effector- to a memory-type response. J. Immunol. 2013;190:2150–2158. doi: 10.4049/jimmunol.1202234. [DOI] [PubMed] [Google Scholar]
- Dahlke C., Lunemann S., Kasonta R., Kreuels B., Schmiedel S., Ly M.L., Fehling S.K., Strecker T., Becker S., Altfeld M., Sow A., Lohse A.W., Munoz-Fontela C., Addo M.M. Comprehensive characterization of cellular immune responses following Ebola virus infection. J Infect Dis. 2017;215(2):287–292. doi: 10.1093/infdis/jiw508. (PMID: 27799354) [DOI] [PubMed] [Google Scholar]
- De Santis O., Audran R., Pothin E., Warpelin-Decrausaz L., Vallotton L., Wuerzner G., Cochet C., Estoppey D., Steiner-Monard V., Lonchampt S., Thierry A.C., Mayor C., Bailer R.T., Mbaya O.T., Zhou Y., Ploquin A., Sullivan N.J., Graham B.S., Roman F., De Ryck I., Ballou W.R., Kieny M.P., Moorthy V., Spertini F., Genton B. Safety and immunogenicity of a chimpanzee adenovirus-vectored Ebola vaccine in healthy adults: a randomised, double-blind, placebo-controlled, dose-finding, phase 1/2a study. Lancet Infect. Dis. 2016;16:311–320. doi: 10.1016/S1473-3099(15)00486-7. [DOI] [PubMed] [Google Scholar]
- De Wit E., Marzi A., Bushmaker T., Brining D., Scott D., Richt J.A., Geisbert T.W., Feldmann H. Safety of recombinant VSV-Ebola virus vaccine vector in pigs. Emerg. Infect. Dis. 2015;21:702–704. doi: 10.3201/eid2104.142012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooq F., Beck K., Paolino K.M., Phillips R., Waters N.C., Regules J.A., Bergmann-Leitner E.S. Circulating follicular T helper cells and cytokine profile in humans following vaccination with the rVSV-ZEBOV Ebola vaccine. Sci. Rep. 2016;6:27944. doi: 10.1038/srep27944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann H., Jones S.M., Daddario-Dicaprio K.M., Geisbert J.B., Stroher U., Grolla A., Bray M., Fritz E.A., Fernando L., Feldmann F., Hensley L.E., Geisbert T.W. Effective post-exposure treatment of Ebola infection. PLoS Pathog. 2007;3:e2. doi: 10.1371/journal.ppat.0030002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisbert T.W., Daddario-Dicaprio K.M., Geisbert J.B., Reed D.S., Feldmann F., Grolla A., Stroher U., Fritz E.A., Hensley L.E., Jones S.M., Feldmann H. Vesicular stomatitis virus-based vaccines protect nonhuman primates against aerosol challenge with Ebola and Marburg viruses. Vaccine. 2008;26:6894–6900. doi: 10.1016/j.vaccine.2008.09.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisbert T.W., Feldmann H. Recombinant vesicular stomatitis virus-based vaccines against Ebola and Marburg virus infections. J. Infect. Dis. 2011;204(Suppl. 3):S1075–S1081. doi: 10.1093/infdis/jir349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta M., Greer P., Mahanty S., Shieh W.J., Zaki S.R., Ahmed R., Rollin P.E. CD8-mediated protection against Ebola virus infection is perforin dependent. J. Immunol. 2005;174:4198–4202. doi: 10.4049/jimmunol.174.7.4198. [DOI] [PubMed] [Google Scholar]
- Henao-Restrepo A.M., Camacho A., Longini I.M., Watson C.H., Edmunds W.J., Egger M., Carroll M.W., Dean N.E., Diatta I., Doumbia M., Draguez B., Duraffour S., Enwere G., Grais R., Gunther S., Gsell P.S., Hossmann S., Watle S.V., Konde M.K., Keita S., Kone S., Kuisma E., Levine M.M., Mandal S., Mauget T., Norheim G., Riveros X., Soumah A., Trelle S., Vicari A.S., Rottingen J.A., Kieny M.P. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ca Suffit!) Lancet. 2017;389(10068):505–518. doi: 10.1016/S0140-6736(16)32621-6. (Epub 2016 Dec 23.PMID:28017403) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S.M., Feldmann H., Stroher U., Geisbert J.B., Fernando L., Grolla A., Klenk H.D., Sullivan N.J., Volchkov V.E., Fritz E.A., Daddario K.M., Hensley L.E., Jahrling P.B., Geisbert T.W. Live attenuated recombinant vaccine protects nonhuman primates against Ebola and Marburg viruses. Nat. Med. 2005;11:786–790. doi: 10.1038/nm1258. [DOI] [PubMed] [Google Scholar]
- Jones S.M., Stroher U., Fernando L., Qiu X., Alimonti J., Melito P., Bray M., Klenk H.D., Feldmann H. Assessment of a vesicular stomatitis virus-based vaccine by use of the mouse model of Ebola virus hemorrhagic fever. J Infect Dis. 2007;196(Suppl. 2):S404–S412. doi: 10.1086/520591. [DOI] [PubMed] [Google Scholar]
- Kang T.H., Bae H.C., Kim S.H., Seo S.H., Son S.W., Choi E.Y., Seong S.Y., Kim T.W. Modification of dendritic cells with interferon-gamma-inducible protein-10 gene to enhance vaccine potency. J. Gene Med. 2009;11:889–898. doi: 10.1002/jgm.1371. [DOI] [PubMed] [Google Scholar]
- Khurana S., Fuentes S., Coyle E.M., Ravichandran S., Davey R.T., Jr. & Beigel, J. H. Human antibody repertoire after VSV-Ebola vaccination identifies novel targets and virus-neutralizing IgM antibodies. Nat. Med. 2016;22:1439–1447. doi: 10.1038/nm.4201. [DOI] [PubMed] [Google Scholar]
- Krahling V., Becker D., Rohde C., Eickmann M., Eroglu Y., Herwig A., Kerber R., Kowalski K., Vergara-Alert J., Becker S. Development of an antibody capture ELISA using inactivated Ebola Zaire Makona virus. Med. Microbiol. Immunol. 2016;205:173–183. doi: 10.1007/s00430-015-0438-6. [DOI] [PubMed] [Google Scholar]
- Lai L., Davey R., Beck A., Xu Y., Suffredini A.F., Palmore T., Kabbani S., Rogers S., Kobinger G., Alimonti J., Link C.J., Jr., Rubinson L., Stroher U., Wolcott M., Dorman W., Uyeki T.M., Feldmann H., Lane H.C., Mulligan M.J. Emergency postexposure vaccination with vesicular stomatitis virus-vectored Ebola vaccine after needlestick. JAMA. 2015;313:1249–1255. doi: 10.1001/jama.2015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzi A., Engelmann F., Feldmann F., Haberthur K., Shupert W.L., Brining D., Scott D.P., Geisbert T.W., Kawaoka Y., Katze M.G., Feldmann H., Messaoudi I. Antibodies are necessary for rVSV/ZEBOV-GP-mediated protection against lethal Ebola virus challenge in nonhuman primates. Proc. Natl. Acad. Sci. U. S. A. 2013;110:1893–1898. doi: 10.1073/pnas.1209591110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzi A., Hanley P.W., Haddock E., Martellaro C., Kobinger G., Feldmann H. Efficacy of vesicular stomatitis virus-Ebola virus postexposure treatment in rhesus macaques infected with Ebola virus Makona. J. Infect. Dis. 2016;214:S360–s366. doi: 10.1093/infdis/jiw218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzi A., Robertson S.J., Haddock E., Feldmann F., Hanley P.W., Scott D.P., Strong J.E., Kobinger G., Best S.M., Feldmann H. EBOLA VACCINE. VSV-EBOV rapidly protects macaques against infection with the 2014/15 Ebola virus outbreak strain. Science. 2015;349:739–742. doi: 10.1126/science.aab3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcelroy A.K., Akondy R.S., Davis C.W., Ellebedy A.H., Mehta A.K., Kraft C.S., Lyon G.M., Ribner B.S., Varkey J., Sidney J., Sette A., Campbell S., Stroher U., Damon I., Nichol S.T., Spiropoulou C.F., Ahmed R. Human Ebola virus infection results in substantial immune activation. Proc. Natl. Acad. Sci. U. S. A. 2015;112:4719–4724. doi: 10.1073/pnas.1502619112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M., Garron T., Lubaki N.M., Mire C.E., Fenton K.A., Klages C., Olinger G.G., Geisbert T.W., Collins P.L., Bukreyev A. Aerosolized Ebola vaccine protects primates and elicits lung-resident T cell responses. J. Clin. Invest. 2015;125:3241–3255. doi: 10.1172/JCI81532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J.D., Van Der Most R.G., Akondy R.S., Glidewell J.T., Albott S., Masopust D., Murali-Krishna K., Mahar P.L., Edupuganti S., Lalor S., Germon S., Del Rio C., Mulligan M.J., Staprans S.I., Altman J.D., Feinberg M.B., Ahmed R. Human effector and memory CD8 + T cell responses to smallpox and yellow fever vaccines. Immunity. 2008;28:710–722. doi: 10.1016/j.immuni.2008.02.020. [DOI] [PubMed] [Google Scholar]
- Nakaya H.I., Wrammert J., Lee E.K., Racioppi L., Marie-Kunze S., Haining W.N., Means A.R., Kasturi S.P., Khan N., Li, G. M., Mccausland, M., Kanchan, V., Kokko, K. E., Li, S., Elbein, R., Mehta, A. K., Aderem, A., Subbarao, K., Ahmed, R. & Pulendran, B. Systems biology of vaccination for seasonal influenza in humans. Nat. Immunol. 2011;12:786–795. doi: 10.1038/ni.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavot V. Ebola virus vaccines: where do we stand? Clin. Immunol. 2016;173:44–49. doi: 10.1016/j.clim.2016.10.016. [DOI] [PubMed] [Google Scholar]
- Qiu X., Fernando L., Alimonti J.B., Melito P.L., Feldmann F., Dick D., Stroher U., Feldmann H., Jones S.M. Mucosal immunization of cynomolgus macaques with the VSVDeltaG/ZEBOVGP vaccine stimulates strong ebola GP-specific immune responses. PLoS One. 2009;4:e5547. doi: 10.1371/journal.pone.0005547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regules J.A., Beigel J.H., Paolino K.M., Voell J., Castellano A.R., Hu Z., Munoz P., Moon J.E., Ruck R.C., Bennett J.W., Twomey P.S., Gutierrez R.L., Remich S.A., Hack H.R., Wisniewski M.L., Josleyn M.D., Kwilas S.A., Van Deusen N., Mbaya O.T., Zhou Y., Stanley D.A., Jing W., Smith K.S., Shi M., Ledgerwood J.E., Graham B.S., Sullivan N.J., Jagodzinski L.L., Peel S.A., Alimonti J.B., Hooper J.W., Silvera P.M., Martin B.K., Monath T.P., Ramsey W.J., Link C.J., Lane H.C., Michael N.L., Davey R.T., Jr., Thomas S.J. A recombinant vesicular stomatitis virus Ebola vaccine. N. Engl. J. Med. 2017;376:330–341. doi: 10.1056/NEJMoa1414216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruibal P., Oestereich L., Ludtke A., Becker-Ziaja B., Wozniak D.M., Kerber R., Korva M., Cabeza-Cabrerizo M., Bore J.A., Koundouno F.R., Duraffour S., Weller R., Thorenz A., Cimini E., Viola D., Agrati C., Repits J., Afrough B., Cowley L.A., Ngabo D., Hinzmann J., Mertens M., Vitoriano I., Logue C.H., Boettcher J.P., Pallasch E., Sachse A., Bah A., Nitzsche K., Kuisma E., Michel J., Holm T., Zekeng E.G., Garcia-Dorival I., Wolfel R., Stoecker K., Fleischmann E., Strecker T., Di Caro A., Avsic-Zupanc T., Kurth A., Meschi S., Mely S., Newman E., Bocquin A., Kis Z., Kelterbaum A., Molkenthin P., Carletti F., Portmann J., Wolff S., Castilletti C., Schudt G., Fizet A., Ottowell L.J., Herker E., Jacobs T., Kretschmer B., Severi E., Ouedraogo N., Lago M., Negredo A., Franco L., Anda P., Schmiedel S., Kreuels B., Wichmann D., Addo M.M., Lohse A.W., De Clerck H., Nanclares C., Jonckheere S., Van Herp M., Sprecher A., Xiaojiang G., Carrington M., Miranda O., Castro C.M., Gabriel M., Drury P., Formenty P., Diallo B., Koivogui L., Magassouba N., Carroll M.W., Gunther S., Munoz-Fontela C. Unique human immune signature of Ebola virus disease in Guinea. Nature. 2016;533:100–104. doi: 10.1038/nature17949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan N.J., Geisbert T.W., Geisbert J.B., Xu, L., Yang, Z. Y., Roederer, M., Koup, R. A., Jahrling, P. B. & Nabel, G. J. Accelerated vaccination for Ebola virus haemorrhagic fever in non-human primates. Nature. 2003;424:681–684. doi: 10.1038/nature01876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan N.J., Martin J.E., Graham B.S., Nabel G.J. Correlates of protective immunity for Ebola vaccines: implications for regulatory approval by the animal rule. Nat. Rev. Microbiol. 2009;7:393–400. doi: 10.1038/nrmicro2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J.A., Hart M.K. Protection from Ebola virus mediated by cytotoxic T lymphocytes specific for the viral nucleoprotein. J. Virol. 2001;75:2660–2664. doi: 10.1128/JVI.75.6.2660-2664.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong G., Audet J., Fernando L., Fausther-Bovendo H., Alimonti J.B., Kobinger G.P., Qiu X. Immunization with vesicular stomatitis virus vaccine expressing the Ebola glycoprotein provides sustained long-term protection in rodents. Vaccine. 2014;32:5722–5729. doi: 10.1016/j.vaccine.2014.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong G., Richardson J.S., Pillet S., Patel A., Qiu X., Alimonti J., Hogan J., Zhang Y., Takada A., Feldmann H., Kobinger G.P. Immune parameters correlate with protection against ebola virus infection in rodents and nonhuman primates. Sci. Transl. Med. 2012;4 doi: 10.1126/scitranslmed.3004582. (158ra146) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1
2