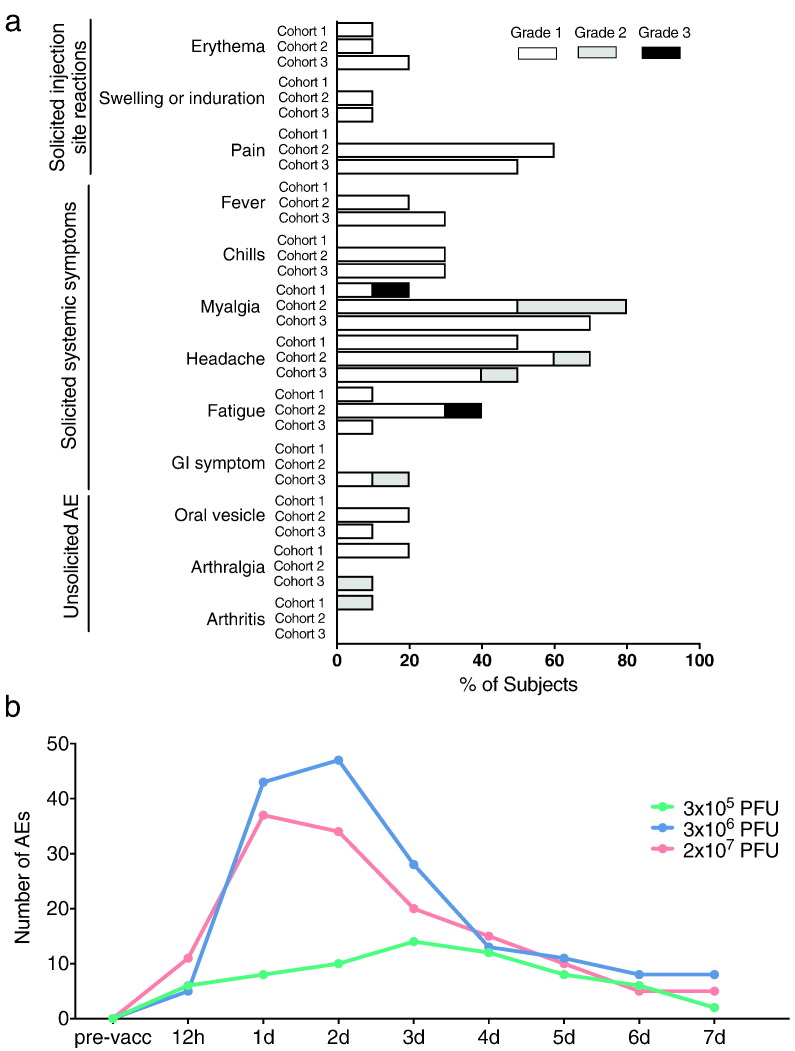
Fig. 2.
Local and systemic adverse events. a) Recorded adverse events of vaccinees during 180 days post vaccination. Events are depicted in frequency (%) of participants among respective cohort presenting at least one adverse event of the category. Adverse events with the severity of mild, moderate and severe are grouped into grade 1, 2 and 3, respectively. Cohort 1: 3 × 105 PFU, Cohort 2: 3 × 106 PFU, Cohort 3: 2 × 107 PFU. b) Number of related solicited and unsolicited adverse events reported over time in the 3 × 105 PFU (cohort 1 (green)), 3 × 106 PFU (cohort 2 (blue)), and 2 × 107 PFU (cohort 3 (red)) dose group.