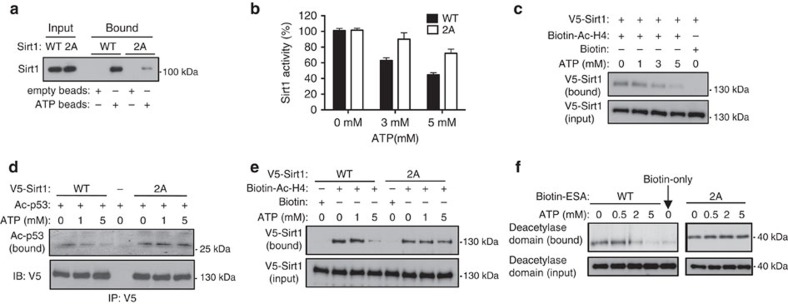
Figure 3. ATP interferes with CTD function.
(a) His-tagged WT or 2A mutant Sirt1 was incubated with either agarose or ATP-agarose beads and bound Sirt1 was visualized by immunoblotting. (b) The catalytic activity of recombinant 2A mutant Sirt1 is less sensitive to ATP. The deacetylation of Ac-H4 by recombinant WT or 2A mutant Sirt1 in the presence of 0–5 mM ATP (n=6). Comparisons between the treatment groups were analysed by two-tailed unpaired Student's t-test. (c) ATP reduces Sirt1-substrate interaction. Biotinylated Ac-H4 peptide was immobilized to streptavidin beads and incubated with V5-tagged Sirt1 in the presence of the indicated concentrations of ATP. Substrate-bound V5-tagged Sirt1 was visualized by immunoblotting with anti-V5 antibody. Biotin immobilized to streptavidin beads without Ac-H4 was used as a negative control. (d) Ac-p53 interaction with 2A Sirt1 is less sensitive to ATP than with WT Sirt1. After incubating V5-tagged WT or 2A Sirt1 with Ac-p53 in the presence of the indicated concentrations of ATP, Sirt1 was pulled down with anti-V5 antibody. Ac-p53 bound to either WT or 2A Sirt1 is shown. For the negative control, we incubated Ac-p53 with no Sirt1. (e) Ac-H4 interaction with 2A Sirt1 is less sensitive to ATP than with WT Sirt1. Ac-H4 interaction with Sirt1 with increasing concentrations of ATP was visualized as in a except 2A Sirt1 was also included. (f) The interaction between the deacetylase domain and the ESA peptide is ATP-sensitive. Deacetylase domain pull-down experiments were performed after streptavidin-immobilized WT or 2A mutant ESA peptides were incubated with recombinant deacetylase domain (a.a. 236–490) in the presence of 0–5 mM ATP. The amount of the deacetylase domain pulled down is shown. Almost no deacetylase domain was pulled down with streptavidin resin alone.