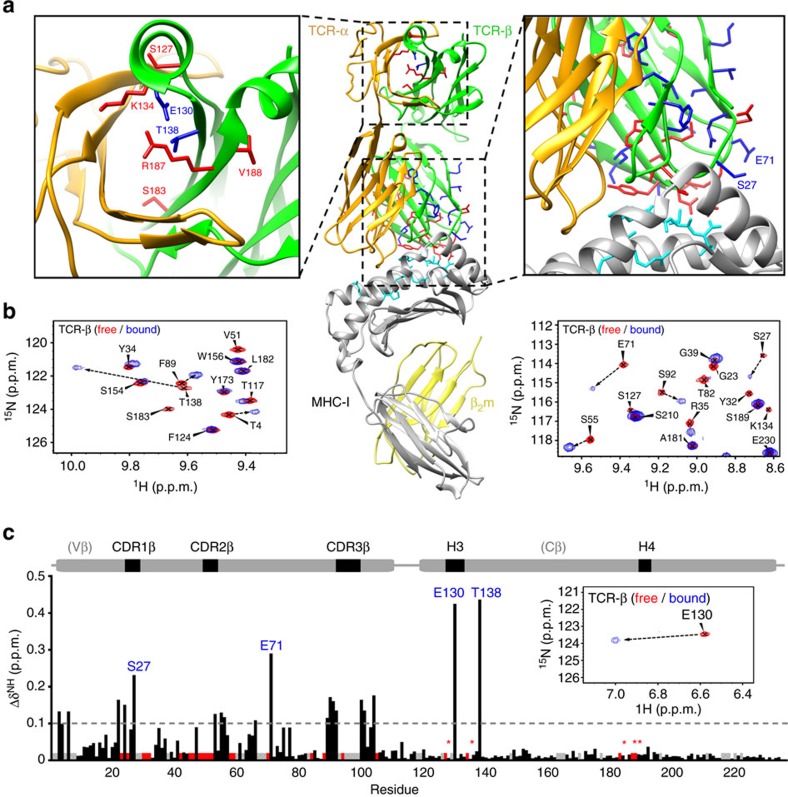
Figure 5. pMHC-induced conformational changes on the TCR β-chain domains.
(a) TCR β-chain residues with significant amide 15N-1H TROSY-HSQC chemical shift changes upon MHC-I binding are shown as sticks on the Cβ and Vβ domains of the αβ-TCR structure. Two types of observed changes are highlighted with different colours: residues with significantly attenuated resonances (>1 s.d. from the average) in the bound form are shown in red, while residues with significant chemical shift changes are in blue. In the middle panel, the tertiary structure of the B4.2.3 (beige/green)/H2-Dd (grey) complex is shown with β2m (yellow) and p18-I10-bound peptide (cyan sticks). (b) Overlays of representative regions from TROSY-HSQC spectra recorded in the free (red) and 1:1 MHC-bound (blue) state of the TCR, using two samples that were 15N, 2H, 13C-labelled at the β-chain and unlabelled α-chain (as shown in Fig. 3a,b). Pairs of peaks that correspond to the same residues in the free and bound forms are connected by dotted arrows. (c) Summary of combined 15N/1H chemical shift changes along the β-chain sequence, scaled relative to 1H. The dashed line signifies 1 s.d. of combined chemical shift change from the average. Residues with significantly attenuated resonances in the bound form are shown as red bars, while unassigned and Pro residues are in grey. Red stars highlight Cβ domain resonances that disappear upon p/MHC binding, and that are also illustrated in the structure diagram above (a). The large chemical shift change observed for Glu130 upon p/MHC binding is highlighted in the inset.