Fig. 4.
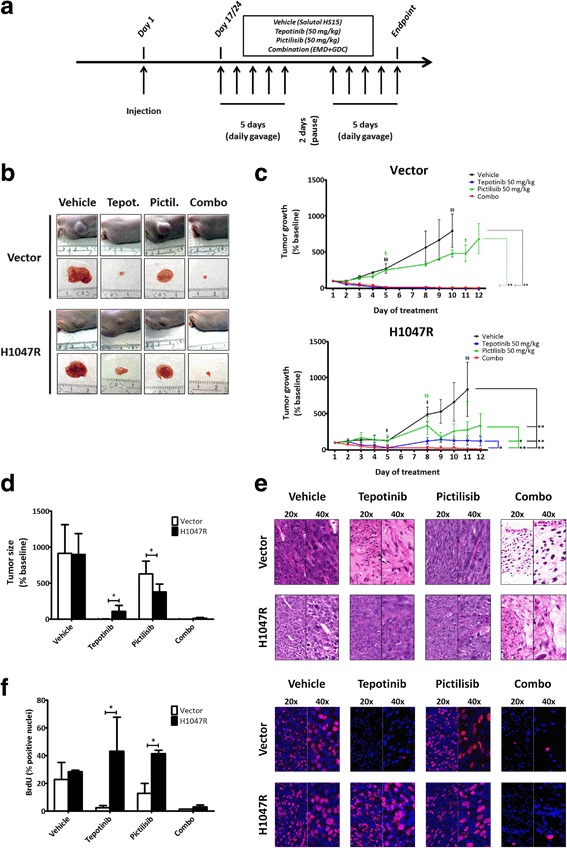
Presence of PIK3CAH1047R mutation confers resistance to MET-targeted inhibition in a subcutaneous xenograft model. a, in vivo experimental design. Nude mice were treated via oral gavage with tepotinib 50 mg/kg alone, pictilisib 50 mg/kg alone, or combination of both drugs for 5 consecutive days, followed by a hiatus of 2 days, and 5 more days of treatment. Mice were euthanized 4 h after the last oral gavage. b, representative images at experimental endpoint of tumors in situ and immediately after extraction. c, tumor growth delay curves of xenografts generated with vector and H1047R cells (“§” indicates early interruptions due to tumor size and/or bleeding). Daily sizes of individual tumors were normalized to their size at the beginning of treatment (here reported as size relative to baseline ± SD). d, comparison of tumor sizes in the four treatment conditions at endpoint. e, morphological assessment of tumors by H&E staining. f, BrdU uptake in vivo and quantification of positive nuclei (quantification in vector-transfected group/combination treatment: only one tumor could be evaluated due to a loss of cellular content in the other tumors; p-value not calculated)
