Abstract
Rationale: The development of host-targeted, prophylactic, and therapeutic interventions against tuberculosis requires a better understanding of the immune mechanisms that determine the outcome of infection with Mycobacterium tuberculosis.
Objectives: To identify T-cell–dependent mechanisms that are protective in tuberculosis.
Methods: Multicolor flow cytometry, cell sorting and growth inhibition assays were employed to compare the frequency, phenotype and function of T lymphocytes from bronchoalveolar lavage or the peripheral blood.
Measurements and Main Results: At two independent study sites, bronchoalveolar lavage cells from donors with latent tuberculosis infection limited the growth of virulent Mycobacterium tuberculosis more efficiently than those in patients who developed disease. Unconventional, glycolipid-responsive T cells contributed to reduced mycobacterial growth because antibodies to CD1b inhibited this effect by 55%. Lipoarabinomannan was the most potent mycobacterial lipid antigen (activation of 1.3% T lymphocytes) and activated CD1b-restricted T cells that limited bacterial growth. A subset of IFN-γ–producing lipoarabinomannan-responsive T cells coexpressed the cytotoxic molecules perforin, granulysin, and granzyme B, which we termed polycytotoxic T cells. Taking advantage of two well-defined cohorts of subjects latently infected with Mycobacterium tuberculosis or patients who developed active disease after infection, we found a correlation between the frequency of polycytotoxic T cells and the ability to control infection (latent tuberculosis infection, 62%; posttuberculosis patients, 26%).
Conclusions: Our data define an unconventional CD8+ T-cell subset (polycytotoxic T cells) that is based on antigen recognition and function. The results link clinical and mechanistic evidence that glycolipid-responsive, polycytotoxic T cells contribute to protection against tuberculosis.
Keywords: cytotoxicity, glycolipid antigens, infectious immunology, unconventional T cells
At a Glance Commentary
Scientific Knowledge on the Subject
The development of host-targeted, prophylactic, and therapeutic interventions against tuberculosis requires a better understanding of the immune mechanisms that determine the outcome of infection with Mycobacterium tuberculosis.
What This Study Adds to the Field
Our data define a novel CD8+ T-cell subset, termed polycytotoxic T cells, based on antigen recognition and function, which contributes to protection against tuberculosis in humans. The findings should encourage the search for strategies that target the expansion and functionality of glycolipid-responsive, antimicrobial CD8+ T cells toward engendering protective immunity in tuberculosis.
Protection against infection with Mycobacterium tuberculosis (Mtb) is dependent on a balanced interaction between the innate and the adaptive immune systems. After inhalation of mycobacteria-containing droplets, one group of individuals will clear the infection by innate immune responses (1). In a second group, elimination of Mtb requires activation of macrophages by antigen-specific effector T cells as part of the adaptive immune response by secretion of cytokines, cytotoxicity, or release of antimicrobial peptides. These subjects remain healthy and will maintain a pool of Mtb-specific memory cells that can be identified ex vivo by IFN-γ release following Mtb antigen–specific stimulation. A positive IFN-γ release assay in healthy individuals with no history of active tuberculosis infection defines latent tuberculosis infection (LTBI) (2). Individuals with LTBI successfully control mycobacterial infection and are protected from the development of active disease (3, 4). Less than 5% of infected individuals will develop active tuberculosis during the following years if innate and adaptive immunity fail to control the proliferation of Mtb (5).
Because of the critical role of T cells in protecting against tuberculosis, the investigation of T-cell recognition of Mtb antigens and subsequent functional responses are of fundamental importance. Much has been learned from the study of classical T-cell responses involving the recognition of peptide antigens in the context of major histocompatibility complex (MHC) class I and MHC class II molecules. However, in experimental animal studies and a recent human vaccine trial, CD4+ T cells producing IFN-γ appeared to be necessary but not sufficient for protection in tuberculosis, and the role of conventional CD8+ T cells remains unclear. Evidence is accumulating that nonclassical antigen-presenting molecules such as the MHC class I–like molecules HLA-E (6–8), MR1 (9), or group 1 CD1 molecules (10) extend the spectrum and biochemical diversity of antigens recognized by the T-cell receptor. The unique ultrastructure of group 1 CD1 molecules (CD1a, CD1b, and CD1c) facilitates binding to lipophilic compounds that can subsequently be presented to T cells. Mycobacterial lipid antigens identified to date include mycolic acids (11), lipoarabinomannan (LAM), phosphatidyl inositol mannan (PIM) (12), glucose monomycolate (GMM) (13), diacylated sulfoglycolipid (Ac2SGL) (14), dideoxymycobactin (15), and glycerol monomycolate (GroMM) (16). Functionally, CD1-restricted T cells have been shown to activate macrophages by the release of Th1 cytokines and to mediate an antimicrobial pathway dependent on the granular proteins perforin and granulysin (17, 18).
A major obstacle in clarifying the in vivo relevance of CD1-restricted T cells is the lack of group 1 CD1 molecules in mice, the prevalent animal model for studying immune responses in tuberculosis. Even though lipid- and glycolipid-specific T cells can be readily detected in Mtb-primed individuals (10) and patients with tuberculosis even years after recovery (19), in-depth functional studies of this subset in clinical cohorts are lacking. The aim of this study was therefore to evaluate the functional relevance of CD1-restricted T cells to protect humans from tuberculosis in a large and well-defined cohort of LTBI donors or patients with tuberculosis after completion of therapy (post-TB). We identified LAM-responsive T cells that coexpress perforin, granzyme B, and granulysin (“polycytotoxic T cells”) as an antimicrobial effector subset in human tuberculosis. The frequency of lipid-responsive polycytotoxic T cells correlated with protection from active disease, which reinforces the concept of exploiting CD1-restricted lipid antigens for immune prophylaxis and therapy against tuberculosis.
Methods
Study Population
Donors for bronchoalveolar lavage (BAL) and peripheral blood were recruited as part of the prospective, multicenter Pulmonary Tuberculosis—Host and Pathogen Determinants of Resistance and Disease Progression study between 2008 and 2014. Blood donors with LTBI were recruited from two groups (20): (1) health care workers from 18 German pulmonary medicine centers (see online supplement) with regular contact with patients with tuberculosis (>2 yr) and (2) household contact persons who were recruited by health care centers (Frankfurt, Hamburg, and Hannover) and who were exposed for more than 40 hours in total to a patient with smear-positive tuberculosis. Post-TB patients were defined as individuals with a history of pulmonary tuberculosis who completed a standard course of tuberculosis treatment more than 6 months in duration before enrollment. Subjects with known HIV infection were excluded. Healthy contact persons were offered BAL performed according to current German guidelines (21). The study protocol was initially approved by the University of Lübeck ethics committee (reference 07-125) and was adopted by the ethics committees of all participating centers. Written informed content was received from participants before inclusion in the study.
Lipid-Responsive IFN-γ Release by T Cells
Blood samples were used either on the same day of venipuncture (Institute for Clinical Transfusion Medicine and Immunogenetics Ulm) or after overnight delivery from the health care centers and pulmonary medicine clinics. The study did not include frozen samples. CD1+ antigen-presenting cells (APCs) were differentiated from plastic adherent peripheral blood mononuclear cells (PBMCs) by treatment with granulocyte-macrophage colony-stimulating factor (10 μg/ml; Miltenyi Biotec Bergisch Gladbach, Germany) and IL-4 (10 μg/ml; BioLegend, San Diego, CA) for 4 ± 1 days. CD1+ APCs expressed MHC class II (95 ± 6%), CD1a (25 ± 12%), CD1b (64.3 ± 15%), CD1c (84 ± 13%), CD86 (95 ± 5%), CD40 (99 ± 0.4%), and the mannose receptor (92 ± 5%). CD1+ APCs were added to autologous nonadherent cells (1:1 ratio), which had been maintained with IL-2 (10 IU of Proleukin; Novartis, Basel, Switzerland) for 4 ± 1 days. Lipid antigens were added for 18 hours, and the IFN-γ concentration in the supernatants was measured by sandwich ELISA (Thermo Fisher Scientific, Waltham, MA) according to the manufacturer´s instructions (sensitivity, 16–64 pg/ml).
Detection of Polycytotoxic T Cells by Flow Cytometry
CD1+ APCs (5 × 106) were mixed with 15 × 106 nonadherent PBMCs (1:3 ratio). Cultures (4 × 106/well) were incubated in the presence of LAM (10 μg/ml) for 14 hours before brefeldin A (10 μg/ml; Sigma-Aldrich, St. Louis, MO) was added to the cultures for the final 4hours of incubation. Cells were harvested, and antibodies directed against cell surface antigens (CD3, CD4, and CD8) were added for 30 minutes at 4°C. Cells were fixed with paraformaldehyde (2%; Sigma-Aldrich) for 20 minutes on ice in the dark, permeabilized with BD Perm/Wash solution (BD Biosciences, San Jose, CA), and incubated with anti–perforin-fluorescein isothiocyanate (3 μl), anti–granzyme B-APCs (2 μl), and antigranulysin (1 μl of 1:50 diluted rabbit serum). After washing with BD Perm/Wash solution, a biotinylated goat antirabbit antibody (0.5 μl) was added, followed by labeling with streptavidin–phycoerythrin–cyanine 7 (0.5 μl). Cells were immediately analyzed using a BD FACSCanto II system (BD Biosciences) and FlowJo software (FlowJo, Ashland, OR). For the detection of polycytotoxic T cells, at least 5 × 106 cells were acquired per sample.
Statistics
The Mann–Whitney U test or Wilcoxon matched pairs signed-rank test was used to determine statistical significance between study cohorts. Differences were considered significant at P < 0.05.
Results
Effect of BAL Cells from LTBI Donors and Post-TB Patients on the Growth of Mtb
To identify T-cell populations involved in local protection against tuberculosis, we compared the ability of BAL cells to control Mtb growth in two different cohorts (see Table E1 in the online supplement). BAL cells were incubated with virulent Mtb (20), and the number of viable bacilli was determined after 2 hours and 5 days by growth inhibition assay. Mycobacterial multiplication was less pronounced in LTBI donors (n = 7) than in post-TB patients (n = 37) (median, 5.8 vs. 16 Mtb multiplication) (Figure 1A, left panel). A second group of individuals (n = 27) recruited within the same study but at different sites served as a validation cohort. Experiments performed by different researchers in a different laboratory confirmed that BAL cells from LTBI donors (n = 11) were more efficient in limiting Mtb growth than those in post-TB patients (n = 16) (median, 0.62 vs. 4.3 Mtb multiplication) (Figure 1A, right panel). Therefore, the capacity of BAL cells to restrict mycobacterial growth correlated with the ability to prevent the development of active disease after infection with Mtb.
Figure 1.
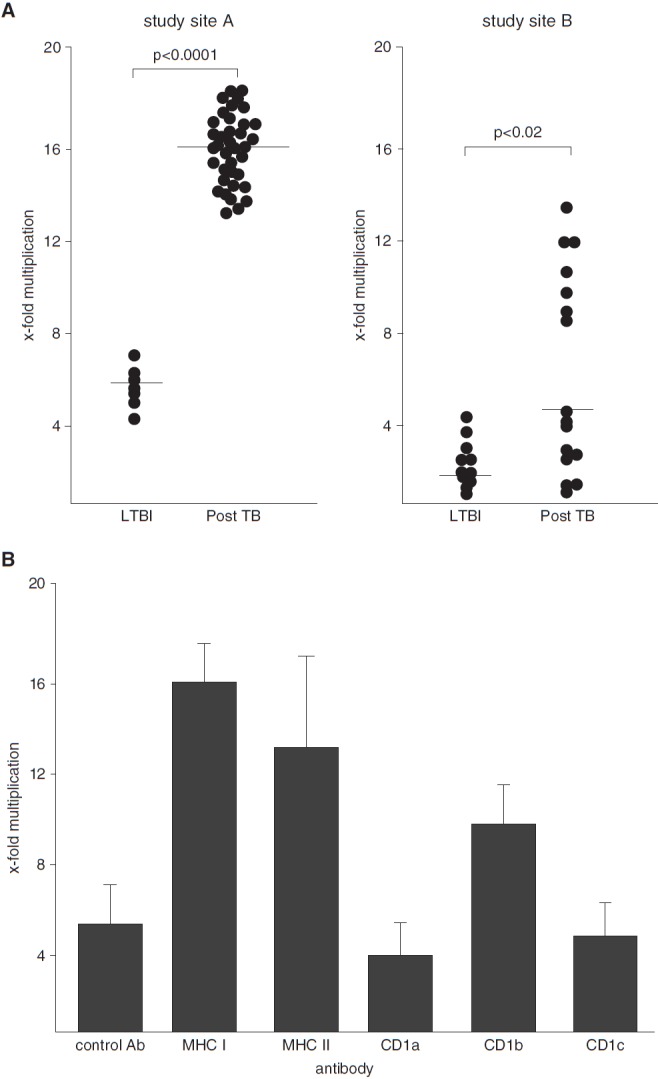
Effect of bronchoalveolar lavage (BAL) cells on the growth of Mycobacterium tuberculosis (Mtb). A total of 2 × 105 freshly isolated BAL cells were incubated with 1 × 106 Mtb cells. The number of colony-forming units was determined 2 hours and 5 days after infection by plating cell lysates. (A) The x-fold multiplication between 2 hours and 5 days of all donors tested is shown. Experiments were performed at different study sites (Ulm [left panel] and Borstel [right panel]). P values were calculated using two-tailed Mann–Whitney U tests. Horizontal bars represent the medians. (B) A total of 2 × 105 BAL cells from donors with latent tuberculosis infection (LTBI) were preincubated with neutralizing antibodies (10 μg/ml) against antigen-presenting molecules for 1 hour before 1 × 106 Mtb cells were added. The number of colony-forming units was determined after 2 hours and 5 days by plating cell lysates. The figure presents the average x-fold multiplication ± SEM from three different donors. Ab = antibody; MHC = major histocompatibility complex; TB = tuberculosis.
Antimicrobial activity is a hallmark of MHC class I–restricted T lymphocytes but has also been associated with CD1-restricted T cells that recognize mycobacterial lipid antigens. To determine the contribution of the respective antigen presentation pathways, we measured the effect of neutralizing antibodies on mycobacterial proliferation in BAL cells of three donors with LTBI in which the cell yield was sufficient. MHC class I–, MHC class II–, and CD1b-restricted T cells were involved in growth inhibition, whereas antibodies directed against CD1a, CD1c, or the isotype control antibody had no effect (Figure 1B). As expected, MHC-restricted T cells were the major antimicrobial subset, but our findings are novel in that that CD1b-dependent T-cell activation contributed to the restriction of Mtb growth in human BAL cells.
Antigen-Specific T-Cell Activation by Purified Mycobacterial Lipids
Few mycobacterial lipids have been shown to be recognized by CD1b-restricted T cells (10). To select for the most immunogenic lipid for detailed mechanistic analysis, we compared the ability of purified LAM, GroMM, Ac2SGL, PIM, and mycolic acid to activate T cells. Because these experiments required a large number of cells, we obtained buffy coat preparations from healthy donors (n = 398) (Table E2), which were screened for the response to PPD by measuring IFN-γ release (Figure E1). PPD+ donors (n = 187 [47%]) were further analyzed for reactivity to mycobacterial lipids in the presence of autologous CD1+ APCs. Preliminary experiments revealed that diluents alone (dimethyl sulfoxide or chloroform/methanol) in concentrations used for these experiments had no effect on the viability or IFN-γ release of T lymphocytes (not shown). The frequency of lipid-responsive T cells was highest at 10 μg/ml, most notably for LAM (1.3% responsive T cells) (Figure 2A), so this concentration was used for the consecutive experiments. Of 187 PPD+ donors, 65 (35%) responded to total lipid, of which 40 (62%) released IFN-γ after stimulation with LAM (Figure 2B). T cells from total lipid–responsive donors were also activated by GroMM (49%), Ac2SGL (36%), PIM (36%), and mycolic acid (22%), albeit to a lesser extent (Figure 2B). Because the frequency of IFN-γ–producing T cells (Figure 2A) and the percentage of reactive donors (Figure 2B) were highest for LAM, we decided to focus on this glycolipid for comparative and mechanistic analyses.
Figure 2.
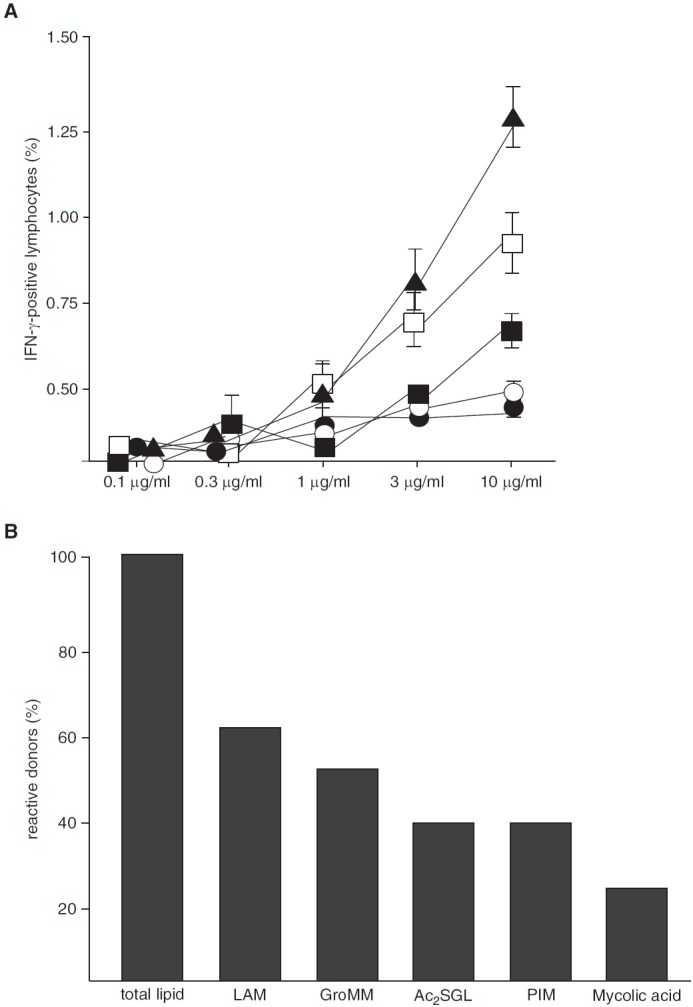
Antigen-specific T-cell activation by purified mycobacterial lipids. (A) Nonadherent peripheral blood mononuclear cells (PBMCs) and autologous CD1+ antigen-presenting cells (APCs) (3:1 ratio) were incubated with purified lipid antigens as indicated (solid triangles, lipoarabinomannan [LAM]; open boxes, glycerol monomycolate [GroMM); solid boxes, diacylated sulfoglycolipid [Ac2SGL]; open circles, phosphatidyl inositol mannan [PIM]; solid circles, mycolic acid). After overnight incubation, brefeldin was added for the final 4 hours of incubation. The frequency of CD3+/IFN-γ+ T cells was determined by flow cytometry. Experiments were performed in triplicates. Error bars show the SD. The figure shows a representative result of five different donors. (B) Nonadherent PBMCs and autologous CD1+ APCs (ratio 3:1) obtained from PPD+ individuals were incubated with total lipid or purified lipid antigens (10 μg/ml). IFN-γ concentration in the supernatant was measured by ELISA after 18 hours. Donors were scored positive if lipid-induced IFN-γ release was threefold greater than the unstimulated control. The number of donors responding to total lipid (n = 65) was set as 100%. The figure gives the percentage of total lipid-responsive donors that responded to the purified lipids.
Frequency of LAM-Responsive T Cells in Healthy Donors
To estimate the contribution of LAM to the immunogenicity of mycobacterial lipids, we compared the frequency of IFN-γ–expressing cells after stimulation with Mtb LAM or total Mtb lipid (both at 10 μg/ml) in 12 healthy donors known to respond to LAM. As expected, the frequency of IFN-γ+ cells was higher for the mixture of total lipids, but LAM alone stimulated nearly half as many T cells (median, 0.9% vs. 1.4%) (Figure 3A). T-cell activation by LAM was almost completely abrogated by neutralizing antibodies to CD1b (Figure 3B), confirming previous findings (12). These results demonstrate that LAM is one of the major T-cell–stimulating antigens, if not the major T-cell–stimulating antigen, within a total lipid extract of Mtb.
Figure 3.
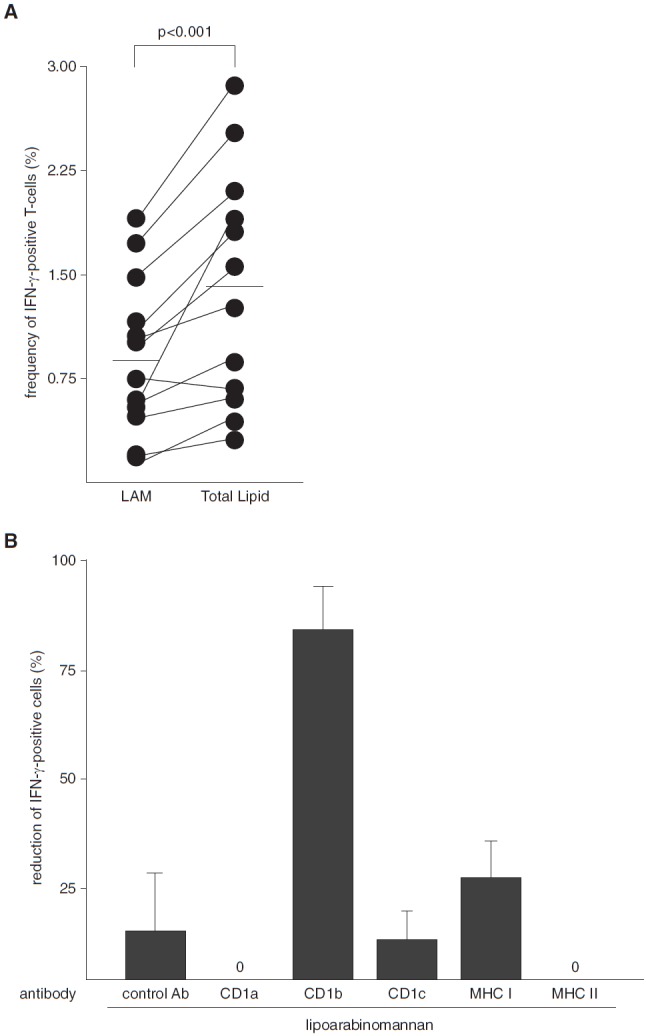
Frequency of lipoarabinomannan (LAM)-responsive T cells in healthy donors. (A) Nonadherent peripheral blood mononuclear cells (PBMCs) and CD1+ antigen-presenting cells (APCs) from LAM-responsive donors were stimulated with LAM (10 μg/ml) or total lipids, and the frequency of CD3+IFN-γ+ T cells was determined after overnight incubation by intracellular flow cytometry. The graph shows the individual results of all 12 donors tested. The P value was calculated using the Wilcoxon matched-pairs signed-rank test. The horizontal bars represent the medians. (B) Nonadherent PBMCs and CD1+ APCs from LAM-responsive donors were preincubated (1 h) with neutralizing antibodies to antigen-presenting molecules as indicated (10 μg/ml each) before LAM (10 μg/ml) was added. After 18 hours of incubation, the concentration of IFN-γ in the supernatants was measured by ELISA. The figure shows the reduction of IFN-γ+ cells as a percentage compared with the samples incubated with LAM in the absence of antibodies. The results are shown as the average ± SEM of three donors. The frequency of LAM-responsive cells in the control cultures was 1.02%. Ab = antibody; MHC = major histocompatibility complex.
LAM-Responsive T Cells Limit the Intracellular Growth of Mtb
Because CD1-restricted T cells contribute to the limitation of Mtb growth by BAL cells (Figure 1B), we hypothesized that LAM activates T cells with antimicrobial activity. PBMCs were stimulated with LAM in the presence of CD1+ APCs, and IFN-γ+ cells were enriched by a magnetic bead-based assay. After 3 days of rest, a growth inhibition assay was performed with LAM-responsive T cells (>70% purity) and Mtb-infected autologous CD1+ APCs. The bacterial load after 48 hours was reduced by LAM-responsive T cells in a dose-dependent manner from 7.9 × 105 cfu (effector/target [E:T] ratio, 0.3:1) to 3.0 × 105 cfu (E:T ratio, 10:1), corresponding to a growth reduction of 62% (Figure 4A, solid circles). In contrast, the IFN-γ− fraction of the same sample did not affect mycobacterial viability (7.7 × 105 cfu at an E:T ratio of 0.3:1 vs. 7.2 × 105 cfu at an E:T ratio of 10:1) (Figure 4A, open circles). Similarly, Mtb readily proliferated in macrophages in the absence of T cells (Figure 4B, solid squares) or in the presence of unsorted nonadherent PBMCs (Figure 4B, open squares), whereas LAM-stimulated IFNγ+ T cells (Figure 4B, solid circles) suppressed the number of viable bacilli below the initial inoculum. Mycobacterial growth was restored by neutralizing antibodies to CD1b, confirming that the effect was dependent on CD1b-restricted T-cell activation (Figure 4C). These in vitro results suggested that LAM-reactive T cells possibly contribute to protection in tuberculosis.
Figure 4.
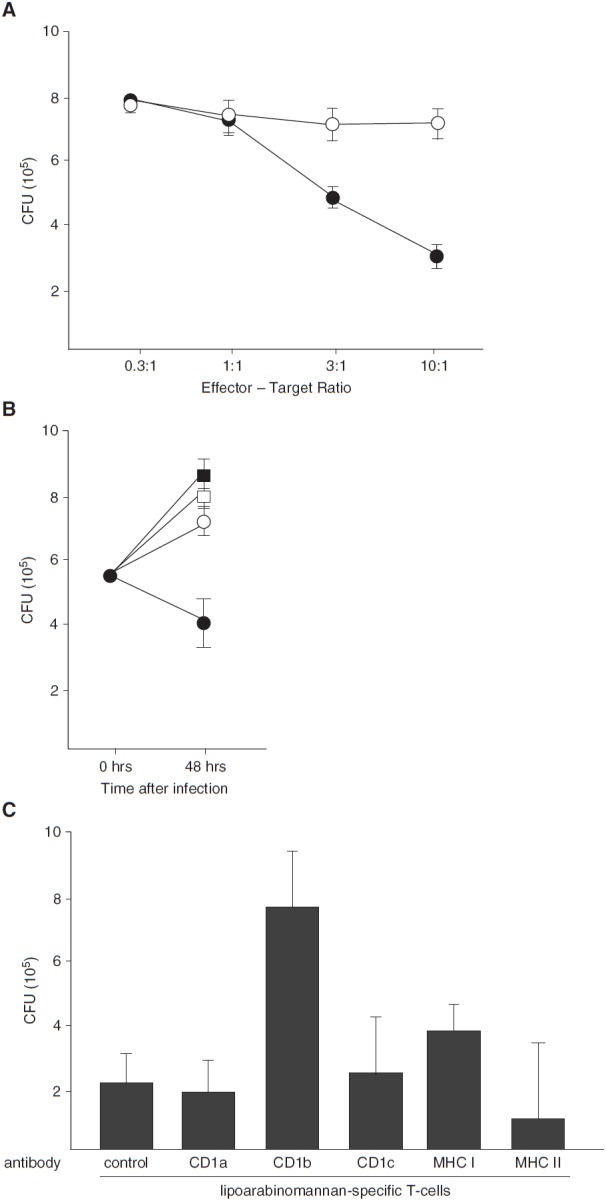
Lipoarabinomannan (LAM)-responsive T cells limit the intracellular growth of Mycobacterium tuberculosis (Mtb). (A) A total of 100 × 106 nonadherent peripheral blood mononuclear cells (PBMCs) of LAM-reactive donors and 30 × 106 autologous CD1+ antigen-presenting cells (APCs) were stimulated with LAM (10 μg/ml), and IFN-γ–secreting cells were enriched by an IFN-γ secretion assay after overnight incubation. Enriched IFN-γ− (open circles) or IFN-γ+ (solid circles) cells were incubated with Mtb-infected (multiplicity of infection [MOI], 5) autologous CD1+ APCs at different effector/target (E:T) ratios as indicated. After 48 hours, the number of viable bacilli was determined by counting the number of colony-forming units (CFU) in cell lysates. The figure presents the average results of four donors ± SEM. (B) Different populations of effector cells (solid squares, no T cells; open squares, nonadherent PBMCs; open circles, LAM-stimulated, IFN-γ−; solid circles, LAM-stimulated, IFN-γ+) were incubated in triplicate with Mtb-infected (MOI, 5) CD1+ APCs (E:T ratio, 10:1). After 48 hours, the number of viable bacilli was determined by counting the number of colony-forming units. The figure shows a representative result of three independent donors. Error bars give the SD of the triplicates for each sample. (C) CD1+ APCs were infected with Mtb (MOI, 5) and preincubated with neutralizing antibodies to antigen-presenting molecules (10 μg/ml) for 1 hour. LAM-responsive, IFN-γ–secreting cells were enriched as in A and added to the cultures (E:T ratio, 10:1) for 48 hours before the bacterial load was determined by counting colony-forming units. The graph presents the average result ± SEM of three different donors. MHC = major histocompatibility complex.
Frequency of LAM-Responsive T Cells in LTBI Donors and Post-TB Patients
In the absence of an appropriate animal model for studying group 1 CD1-mediated immune responses in vivo, we investigated whether the frequency of LAM-reactive T cells correlates with the ability to control Mtb infection in humans. We recruited two cohorts reflecting divergent clinical responses to infection with Mtb (Table E2). The first cohort comprised positive IFN-γ release assay donors in whom infection was controlled after close contact with patients with tuberculosis (LTBI; n = 22). The second cohort included individuals who had been successfully treated for active tuberculosis (post-TB; n = 18). The frequency of IFN-γ–producing, LAM-responsive T cells was similar in both groups (median, 0.61 for LTBI vs. 0.62 for post-TB) (Figure 5), arguing against a correlation between the frequency of LAM-reactive T cells and the outcome of infection with Mtb. Phenotypic analysis of LAM-responsive, IFN-γ+ T cells in six donors (three LTBI and three post-TB) demonstrated that the majority was CD4+ (81 ± 5%) while 12 ± 3% were CD8+ and 8 ± 3% were CD4−CD8− (Figure E2). Even though the study size did not permit statistical evaluation, there was no striking difference between LTBI and post-TB donors.
Figure 5.
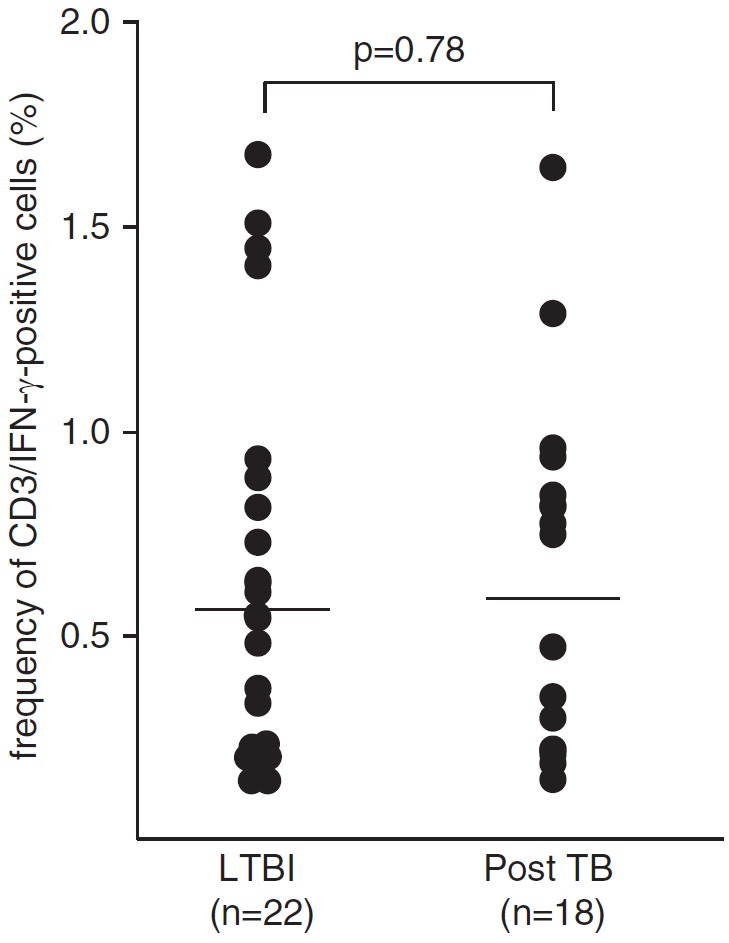
Frequency of lipoarabinomannan (LAM)-responsive T cells in latent tuberculosis infection (LTBI) and posttuberculosis (post-TB) donors. Nonadherent peripheral blood mononuclear cells and autologous CD1+ antigen-presenting cells (3:1 ratio) were incubated with LAM (10 μg/ml). The frequency of CD3+IFN-γ+ T cells was determined after overnight incubation by intracellular flow cytometry. The figure presents the individual results of all donors tested. The P value (0.7751) was calculated using the Mann–Whitney U test for two-tailed samples. The horizontal bars represent the medians.
Frequency and Phenotype of LAM-Responsive Polycytotoxic T Cells in LTBI and Post-TB Donors
Because the frequency and phenotype of LAM-reactive T cells did not discriminate between donors with LTBI and post-TB patients, we hypothesized that functional differences account for the divergent outcome of infection. Given the restriction of Mtb growth by LAM-reactive T cells (Figure 4), we analyzed the expression of perforin, granulysin, and granzyme B, three granular proteins that define a pathway for T-cell–mediated killing of intracellular bacteria (17, 22). PBMCs and autologous CD1+ APCs from LTBI donors (n = 28) or post-TB patients (n = 51) were stimulated overnight with LAM and analyzed by multicolor flow cytometry. CD3+/IFN-γ+ cells were simultaneously stained for granulysin, perforin, and granzyme B as detailed in the online supplement and Figure 6A. LAM-responsive CD3+/IFN-γ+/granulysin+ cells that coexpressed perforin and granzyme B were significantly more frequent (P < 0.0001) in donors with LTBI than in post-TB patients (median, 62 vs. 26%) (Figures 6B and 6C). On the basis of the coexpression of three granular cytolytic effector molecules at the single-cell level, we termed these cells polycytotoxic T cells. Granulysin single-positive and granulysin+/granzyme B+ double-positive cells were more frequent in post-TB patients, whereas as the number of granulysin+/perforin+/granzyme B− cells was equally low in both cohorts (Figure 6B).
Figure 6.
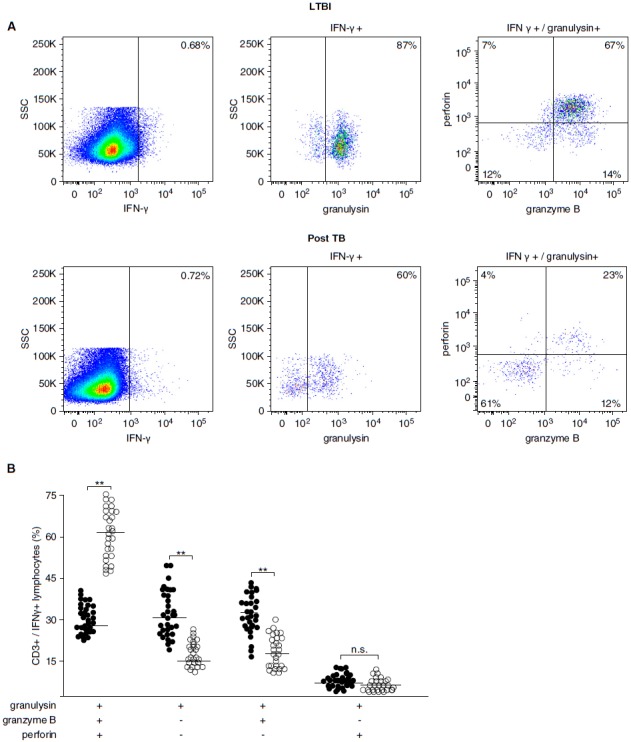
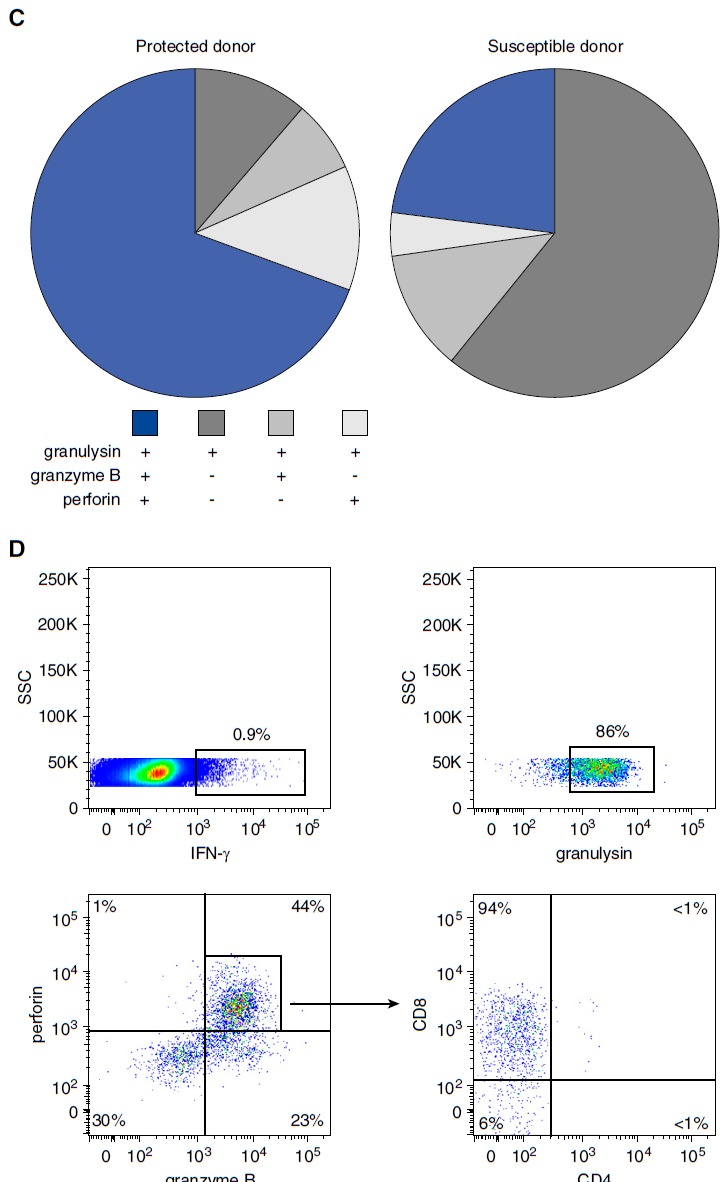
Frequency and phenotype of lipoarabinomannan (LAM)-responsive polycytotoxic T cells in latent tuberculosis infection (LTBI) and posttuberculosis (post-TB) donors. A total of 10 × 106 nonadherent peripheral blood mononuclear cells (PBMCs) and autologous CD1+ antigen-presenting cells (APCs) (3:1 ratio) were stimulated with LAM (10 μg/ml) for 18 hours. Brefeldin was added for the final 4 hours of incubation. Cells were then stained for CD3, IFN-γ, granulysin, granzyme B, and perforin. At least 1 × 106 cells were analyzed by flow cytometry. (A) A representative result for LTBI donors (n = 28) and post-TB patients (n = 51) is shown. (B) The individual results of all donors investigated (solid circles, post-TB; open circles, LTBI) are shown. Statistical significance was calculated using the Mann–Whitney U test (**P < 0.0001). Horizontal bars represent the mean values. (C) The pie charts present a summary of the individual data shown in (B). (D) Nonadherent PBMCs and CD1+ APCs were stimulated as described above, and CD3+/IFN-γ+ polycytotoxic T cells were further characterized by CD4 and CD8 labeling. At least 3 × 106 cells were acquired for each sample. The graph presents one representative result of three different donors. n.s. = not significant; SSC = side scatter.
These results show that LAM-responsive polycytotoxic T cells are more frequent in LTBI donors (potentially protected because they did not develop disease after close contact) than in post-TB patients (potentially susceptible because these patients developed active disease after close contact) (Figure 6C). The coexpression of cytolytic molecules was reminiscent of CD8+CD45RA+ effector memory T cells (TEMRA), which have been implicated in protection against Mtb infection in humans (23, 24). Therefore, we additionally investigated the expression of CD4 and CD8 on the cell surface of LAM-reactive polycytotoxic T cells. Virtually all LAM-responsive polycytotoxic T cells expressed CD8 (Figure 6D). It remains to be determined whether these cells are CD45RA+/CCR7− and define a subset of CD8+ TEMRA.
Discussion
Our understanding of the immune mechanisms that determine the outcome of infection with Mtb in humans, including protection against active disease, is limited. In the present study, we defined a new CD8+ T-cell subset, which we termed polycytotoxic T cells, that is based on antigen recognition and function and may contribute to protection against tuberculosis in humans. The induction of glycolipid-specific CD8+ T-cell responses represents a novel mechanism of protective immunity against tuberculosis.
The finding that polycytotoxic T cells are directly related to disease outcome in tuberculosis indicates that the coexpression of perforin, granzyme B, and granulysin confers an advantage for host defense against Mtb. These cytotoxic granule contents are required for CD8+ T-cell–mediated antimicrobial activity (18). Perforin forms a pore into infected macrophages and delivers granzyme and granulysin into the infected cell. Granulysin itself has direct antimicrobial effects against Mtb (17) by altering the bacterial membrane permeability (25). On the basis of its critical role in mediating antimicrobial activity, granulysin may prove to be a biomarker for protection in tuberculosis. The plasma levels of granulysin correlate with successful treatment of tuberculosis (26, 27), and the expression of granulysin at the site of mycobacterial infection correlates with protection (28, 29) and vaccine efficacy (30). Strikingly, the local delivery of granulysin by a recombinant adenovirus reduced the proliferation of Mtb in mice, highlighting the therapeutic potential of cytotoxic molecules (31).
More recently, it was shown that all three granular proteins are essential in mediating antimicrobial activity against Escherichia coli. In this cell-free model, granulysin perturbs the bacterial cell membrane to permit the entry of granzymes into the cytosol and initiate the production of bactericidal oxygen radicals (22).
The isolation of polycytotoxic T cells requires permeabilization of the cell membrane. Thus, our functional studies had to rely on LAM-responsive T cells that were isolated on the basis of release of IFN-γ. Nevertheless, the correlation between the CD1b-restricted suppression of Mtb growth and the frequency of CD1b-restricted polycytotoxic T cells suggests that this population is responsible for the growth inhibition of Mtb. The identification of a specific pattern of cell surface markers, such as by forward genetic analyses, should facilitate purification of polycytotoxic T cells and lead to a broader understanding of this subset in tuberculosis and other infectious or inflammatory diseases.
While the initial characterization of CD1-restricted human T cells suggested a phenotype comprising CD4−/CD8− and CD8+ T cells (12, 18, 32, 33), the recent development of CD1b tetramers has shed new light on the understanding of glycolipid-reactive T cells in the peripheral blood. GMM-specific T cells express high-affinity, germline-encoded T-cell receptors or more diverse T-cell receptors with low affinity for CD1b–lipid complexes (34). Interestingly, GMM-loaded CD1b tetramers bind predominantly to CD4+ T cells, suggesting that this subset is the major target for mycobacterial glycolipid antigens (35). By using an indirect approach (IFN-γ release), we also found that the majority of glycolipid-responsive T cells in the peripheral blood were CD4+ (Figure E1). Our analysis of IFN-γ+ T cells that coexpress three cytotoxic molecules clearly selected for CD8+ T cells within the heterogeneous population of LAM-responsive T cells (Figure 6B). Therefore, the majority of LAM-responsive T cells in terms of IFN-γ release in the peripheral blood are CD4+, whereas polycytotoxic T cells are confined to the CD8+ compartment.
Previously, we demonstrated that CD8+ TEMRA express granulysin and perforin and exert profound antimycobacterial activity (23). The decreased frequency of these effector T cells correlated with an increased risk for development of severe infections with intracellular bacteria in patients receiving therapy with anti–tumor necrosis factor-α antibodies (36). CD8+ TEMRA responding to mycobacterial protein antigens were also more prevalent in protected donors than in patients with active disease (24). In the present study, the frequency of glycolipid-responsive CD8+ polycytotoxic T cells positively correlated with the ability to prevent active tuberculosis and develop LTBI after intense contact with an index patient. Taken together, these findings provide evidence that a functional subset of CD8+ T cells combining all three cytolytic and antimicrobial effector molecules—perforin, granzyme B, and granulysin—contribute to protection against intracellular bacteria in humans. Our results do not rule out the possibility that polycytotoxic T cells are abundant in post-TB patients before Mtb infection, are then depleted during active disease, and consequently are less frequent after treatment. However, on the basis of recent findings, we hypothesize that 6 months after the completion of therapy, which was our inclusion criterion, the T-cell memory pool will have recovered to the predisease level (37).
In addition to CD1b-restricted T cells, our data indicate a role for MHC class I– and MHC class II–restricted T cells in contributing to the mycobacterial growth inhibition of human bronchoalveolar cells. It remains to be determined whether other CD8+ T-cell populations, including MHC class I– or HLA-E–restricted subsets that are known to exert antimycobacterial activity, are similarly polycytotoxic (8, 18, 38). CD8+ MR1-restricted mucosal-associated invariant T cells lyse Mtb-infected macrophages, produce Th1 cytokines, and are enriched in the respiratory tract of patients with active tuberculosis (39). They express perforin, granzyme B, and granulysin, although it remains to be determined whether there is a distinct polycytotoxic subpopulation simultaneously expressing all three granular proteins.
We believe that these results have significance for understanding the dynamics of clinical tuberculosis and implications for vaccine development. While it is widely believed that Mtb infection does not protect against reinfection, there are compelling data that LTBI can indeed provide up to 80% protection against subsequent disease (3, 4). That raises the question why there is such a high rate of recurrence in patients apparently successfully treated in areas where TB is highly endemic. It is possible that latent infection provides a continuous level of stimulation required for maintaining the pool of protective memory cells, and successful treatment reduces the antigen load below this critical threshold. Thus, post-TB patients may be more appropriately assigned to the susceptible epidemiologic category. That suggests that vaccination of treated patients in high endemic areas may be able to maintain a protective level of immune responses and reduce recurrence of tuberculosis. It further suggests that, in future vaccine trials, inclusion of lipid antigens that can generate polycytotoxic T cells may provide enhanced protection.
In summary, we introduce an unconventional, phenotypically distinct T-cell subset limiting the multiplication of Mtb, the CD8+ polycytotoxic T cells. Our findings should encourage a search for strategies to identify antigens or immune-modulatory compounds that directly target the expansion and functionality of CD8+ polycytotoxic T cells toward engendering protective immunity in human tuberculosis.
Acknowledgments
Acknowledgment
The authors acknowledge the technical assistance of Mark Grieshober and Stefanie Hauk and the support of Dr. Karoline Gaede in managing the TBornotTB Network. The authors thank the Institute for Transfusion Medicine and Immunogenetics, University Hospital Ulm, for supplying buffy coats, as well as the following individuals for supplying mycobacterial antigens and advice regarding the quality control of mycobacterial antigens: Martine Gilleron, INSERM, Institute of Pharmacology and Structural Biology, Toulouse, France; D. B. Moody, Harvard Medical School, Boston, Massachusetts; and John Belisle and Karen Dobos, Colorado State University, Fort Collins, Colorado.
Footnotes
A list of TBornotTB Network contributors may be found in the online supplement.
Supported by the German Ministry for Education und Science (grants 01KI0772 and 01KI0783), the European Union (NewTBVAC and TBVAC2020), and Land Baden Württemberg (Promotionskolleg “Pharmazeutische Biotechnologie”).
Author Contributions: Conception and design: C. Herzmann, C.L., R.L.M., and S.S.; analysis and interpretation: M.B., C. Herzmann, S.K., R.L.M., A.Z., C. Höfer, D.M., S.F.Z., R.M., and S.S.; drafting of the manuscript for important intellectual content: B.R.B., R.L.M., and S.S.; and collection and management of patient samples: TBornotTB Network.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201509-1746OC on February 17, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Salgame P, Geadas C, Collins L, Jones-López E, Ellner JJ. Latent tuberculosis infection–revisiting and revising concepts. Tuberculosis (Edinb) 2015;95:373–384. doi: 10.1016/j.tube.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Mack U, Migliori GB, Sester M, Rieder HL, Ehlers S, Goletti D, Bossink A, Magdorf K, Hölscher C, Kampmann B, et al. C. Lange; TBNET. LTBI: latent tuberculosis infection or lasting immune responses to M. tuberculosis? A TBNET consensus statement. Eur Respir J. 2009;33:956–973. doi: 10.1183/09031936.00120908. [DOI] [PubMed] [Google Scholar]
- 3.Heimbeck J. Incidence of tuberculosis in young adult women, with special reference to employment. Br J Tuberc. 1938;32:154–166. [Google Scholar]
- 4.Andrews JR, Noubary F, Walensky RP, Cerda R, Losina E, Horsburgh CR. Risk of progression to active tuberculosis following reinfection with Mycobacterium tuberculosis. Clin Infect Dis. 2012;54:784–791. doi: 10.1093/cid/cir951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zellweger JP, Sotgiu G, Block M, Dore S, Altet N, Blunschi R, Bogyi M, Bothamley G, Bothe C, Codecasa L, et al. TBNET. Risk assessment of tuberculosis in contacts by IFN-γ release assays: a Tuberculosis Network European Trials Group Study. Am J Respir Crit Care Med. 2015;191:1176–1184. doi: 10.1164/rccm.201502-0232OC. [DOI] [PubMed] [Google Scholar]
- 6.Heinzel AS, Grotzke JE, Lines RA, Lewinsohn DA, McNabb AL, Streblow DN, Braud VM, Grieser HJ, Belisle JT, Lewinsohn DM. HLA-E–dependent presentation of Mtb-derived antigen to human CD8+ T cells. J Exp Med. 2002;196:1473–1481. doi: 10.1084/jem.20020609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joosten SA, van Meijgaarden KE, van Weeren PC, Kazi F, Geluk A, Savage ND, Drijfhout JW, Flower DR, Hanekom WA, Klein MR, et al. Mycobacterium tuberculosis peptides presented by HLA-E molecules are targets for human CD8 T-cells with cytotoxic as well as regulatory activity. PLoS Pathog. 2010;6:e1000782. doi: 10.1371/journal.ppat.1000782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Meijgaarden KE, Haks MC, Caccamo N, Dieli F, Ottenhoff TH, Joosten SA. Human CD8+ T-cells recognizing peptides from Mycobacterium tuberculosis (Mtb) presented by HLA-E have an unorthodox Th2-like, multifunctional, Mtb inhibitory phenotype and represent a novel human T-cell subset. PLoS Pathog. 2015;11:e1004671. doi: 10.1371/journal.ppat.1004671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gold MC, Napier RJ, Lewinsohn DM. MR1-restricted mucosal associated invariant T (MAIT) cells in the immune response to Mycobacterium tuberculosis. Immunol Rev. 2015;264:154–166. doi: 10.1111/imr.12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Rhijn I, Moody DB. CD1 and mycobacterial lipids activate human T cells. Immunol Rev. 2015;264:138–153. doi: 10.1111/imr.12253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beckman EM, Porcelli SA, Morita CT, Behar SM, Furlong ST, Brenner MB. Recognition of a lipid antigen by CD1-restricted αβ+ T cells. Nature. 1994;372:691–694. doi: 10.1038/372691a0. [DOI] [PubMed] [Google Scholar]
- 12.Sieling PA, Chatterjee D, Porcelli SA, Prigozy TI, Mazzaccaro RJ, Soriano T, Bloom BR, Brenner MB, Kronenberg M, Brennan PJ, et al. CD1-restricted T cell recognition of microbial lipoglycan antigens. Science. 1995;269:227–230. doi: 10.1126/science.7542404. [DOI] [PubMed] [Google Scholar]
- 13.Moody DB, Reinhold BB, Guy MR, Beckman EM, Frederique DE, Furlong ST, Ye S, Reinhold VN, Sieling PA, Modlin RL, et al. Structural requirements for glycolipid antigen recognition by CD1b-restricted T cells. Science. 1997;278:283–286. doi: 10.1126/science.278.5336.283. [DOI] [PubMed] [Google Scholar]
- 14.Gilleron M, Stenger S, Mazorra Z, Wittke F, Mariotti S, Böhmer G, Prandi J, Mori L, Puzo G, De Libero G. Diacylated sulfoglycolipids are novel mycobacterial antigens stimulating CD1-restricted T cells during infection with Mycobacterium tuberculosis. J Exp Med. 2004;199:649–659. doi: 10.1084/jem.20031097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moody DB, Young DC, Cheng TY, Rosat JP, Roura-Mir C, O’Connor PB, Zajonc DM, Walz A, Miller MJ, Levery SB, et al. T cell activation by lipopeptide antigens. Science. 2004;303:527–531. doi: 10.1126/science.1089353. [DOI] [PubMed] [Google Scholar]
- 16.Layre E, Collmann A, Bastian M, Mariotti S, Czaplicki J, Prandi J, Mori L, Stenger S, De Libero G, Puzo G, et al. Mycolic acids constitute a scaffold for mycobacterial lipid antigens stimulating CD1-restricted T cells. Chem Biol. 2009;16:82–92. doi: 10.1016/j.chembiol.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Stenger S, Hanson DA, Teitelbaum R, Dewan P, Niazi KR, Froelich CJ, Ganz T, Thoma-Uszynski S, Melián A, Bogdan C, et al. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science. 1998;282:121–125. doi: 10.1126/science.282.5386.121. [DOI] [PubMed] [Google Scholar]
- 18.Stenger S, Mazzaccaro RJ, Uyemura K, Cho S, Barnes PF, Rosat JP, Sette A, Brenner MB, Porcelli SA, Bloom BR, et al. Differential effects of cytolytic T cell subsets on intracellular infection. Science. 1997;276:1684–1687. doi: 10.1126/science.276.5319.1684. [DOI] [PubMed] [Google Scholar]
- 19.Montamat-Sicotte DJ, Millington KA, Willcox CR, Hingley-Wilson S, Hackforth S, Innes J, Kon OM, Lammas DA, Minnikin DE, Besra GS, et al. A mycolic acid-specific CD1-restricted T cell population contributes to acute and memory immune responses in human tuberculosis infection. J Clin Invest. 2011;121:2493–2503. doi: 10.1172/JCI46216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herzmann C, Sotgiu G, Schaberg T, Ernst M, Stenger S, Lange C German TB or Not TB Consortium. Early BCG vaccination is unrelated to pulmonary immunity against Mycobacterium tuberculosis in adults. Eur Respir J. 2014;44:1087–1090. doi: 10.1183/09031936.00086514. [DOI] [PubMed] [Google Scholar]
- 21.Häussinger K, Ballin A, Becker HD, Bölcskei P, Dierkesmann R, Dittrich I, Frank W, Freitag L, Gottschall R, Guschall WR, et al. Working party on Recommendations for Quality Stanards in Endoscopy of the German Society of Pulmonology (Section Endoscopy) [Recommendations for quality standards in bronchoscopy] [in German] Pneumologie. 2004;58:344–356. doi: 10.1055/s-2004-818406. [DOI] [PubMed] [Google Scholar]
- 22.Walch M, Dotiwala F, Mulik S, Thiery J, Kirchhausen T, Clayberger C, Krensky AM, Martinvalet D, Lieberman J. Cytotoxic cells kill intracellular bacteria through granulysin-mediated delivery of granzymes. Cell. 2014;157:1309–1323. doi: 10.1016/j.cell.2014.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bruns H, Meinken C, Schauenberg P, Härter G, Kern P, Modlin RL, Antoni C, Stenger S. Anti-TNF immunotherapy reduces CD8+ T cell-mediated antimicrobial activity against Mycobacterium tuberculosis in humans. J Clin Invest. 2009;119:1167–1177. doi: 10.1172/JCI38482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rozot V, Vigano S, Mazza-Stalder J, Idrizi E, Day CL, Perreau M, Lazor-Blanchet C, Petruccioli E, Hanekom W, Goletti D, et al. Mycobacterium tuberculosis-specific CD8+ T cells are functionally and phenotypically different between latent infection and active disease. Eur J Immunol. 2013;43:1568–1577. doi: 10.1002/eji.201243262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ernst WA, Thoma-Uszynski S, Teitelbaum R, Ko C, Hanson DA, Clayberger C, Krensky AM, Leippe M, Bloom BR, Ganz T, et al. Granulysin, a T cell product, kills bacteria by altering membrane permeability. J Immunol. 2000;165:7102–7108. doi: 10.4049/jimmunol.165.12.7102. [DOI] [PubMed] [Google Scholar]
- 26.Di Liberto D, Buccheri S, Caccamo N, Meraviglia S, Romano A, Di Carlo P, Titone L, Dieli F, Krensky AM, Salerno A. Decreased serum granulysin levels in childhood tuberculosis which reverse after therapy. Tuberculosis (Edinb) 2007;87:322–328. doi: 10.1016/j.tube.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mueller H, Faé KC, Magdorf K, Ganoza CA, Wahn U, Guhlich U, Feiterna-Sperling C, Kaufmann SH. Granulysin-expressing CD4+ T cells as candidate immune marker for tuberculosis during childhood and adolescence. PLoS One. 2011;6:e29367. doi: 10.1371/journal.pone.0029367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ochoa MT, Stenger S, Sieling PA, Thoma-Uszynski S, Sabet S, Cho S, Krensky AM, Rollinghoff M, Nunes Sarno E, Burdick AE, et al. T-cell release of granulysin contributes to host defense in leprosy. Nat Med. 2001;7:174–179. doi: 10.1038/84620. [DOI] [PubMed] [Google Scholar]
- 29.Rahman S, Gudetta B, Fink J, Granath A, Ashenafi S, Aseffa A, Derbew M, Svensson M, Andersson J, Brighenti SG. Compartmentalization of immune responses in human tuberculosis: few CD8+ effector T cells but elevated levels of FoxP3+ regulatory T cells in the granulomatous lesions. Am J Pathol. 2009;174:2211–2224. doi: 10.2353/ajpath.2009.080941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rahman S, Magalhaes I, Rahman J, Ahmed RK, Sizemore DR, Scanga CA, Weichold F, Verreck F, Kondova I, Sadoff J, et al. Prime-boost vaccination with rBCG/rAd35 enhances CD8⁺ cytolytic T-cell responses in lesions from Mycobacterium tuberculosis-infected primates. Mol Med. 2012;18:647–658. doi: 10.2119/molmed.2011.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma J, Lu J, Huang H, Teng X, Tian M, Yu Q, Yuan X, Jing Y, Shi C, Li J, et al. Inhalation of recombinant adenovirus expressing granulysin protects mice infected with Mycobacterium tuberculosis. Gene Ther. 2015;22:968–976. doi: 10.1038/gt.2015.73. [DOI] [PubMed] [Google Scholar]
- 32.Porcelli S, Morita CT, Brenner MB. CD1b restricts the response of human CD4−8−T lymphocytes to a microbial antigen. Nature. 1992;360:593–597. doi: 10.1038/360593a0. [DOI] [PubMed] [Google Scholar]
- 33.Rosat JP, Grant EP, Beckman EM, Dascher CC, Sieling PA, Frederique D, Modlin RL, Porcelli SA, Furlong ST, Brenner MB. CD1-restricted microbial lipid antigen-specific recognition found in the CD8+ αβ T cell pool. J Immunol. 1999;162:366–371. [PubMed] [Google Scholar]
- 34.Van Rhijn I, Kasmar A, de Jong A, Gras S, Bhati M, Doorenspleet ME, de Vries N, Godfrey DI, Altman JD, de Jager W, et al. A conserved human T cell population targets mycobacterial antigens presented by CD1b. Nat Immunol. 2013;14:706–713. doi: 10.1038/ni.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kasmar AG, van Rhijn I, Cheng TY, Turner M, Seshadri C, Schiefner A, Kalathur RC, Annand JW, de Jong A, Shires J, et al. CD1b tetramers bind αβ T cell receptors to identify a mycobacterial glycolipid-reactive T cell repertoire in humans. J Exp Med. 2011;208:1741–1747. doi: 10.1084/jem.20110665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Solovic I, Sester M, Gomez-Reino JJ, Rieder HL, Ehlers S, Milburn HJ, Kampmann B, Hellmich B, Groves R, Schreiber S, et al. The risk of tuberculosis related to tumour necrosis factor antagonist therapies: a TBNET consensus statement. Eur Respir J. 2010;36:1185–1206. doi: 10.1183/09031936.00028510. [DOI] [PubMed] [Google Scholar]
- 37.Bloom CI, Graham CM, Berry MP, Wilkinson KA, Oni T, Rozakeas F, Xu Z, Rossello-Urgell J, Chaussabel D, Banchereau J, et al. Detectable changes in the blood transcriptome are present after two weeks of antituberculosis therapy. PLoS One. 2012;7:e46191. doi: 10.1371/journal.pone.0046191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cho S, Mehra V, Thoma-Uszynski S, Stenger S, Serbina N, Mazzaccaro RJ, Flynn JL, Barnes PF, Southwood S, Celis E, et al. Antimicrobial activity of MHC class I-restricted CD8+ T cells in human tuberculosis. Proc Natl Acad Sci USA. 2000;97:12210–12215. doi: 10.1073/pnas.210391497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Le Bourhis L, Dusseaux M, Bohineust A, Bessoles S, Martin E, Premel V, Coré M, Sleurs D, Serriari NE, Treiner E, et al. MAIT cells detect and efficiently lyse bacterially-infected epithelial cells. PLoS Pathog. 2013;9:e1003681. doi: 10.1371/journal.ppat.1003681. [DOI] [PMC free article] [PubMed] [Google Scholar]


