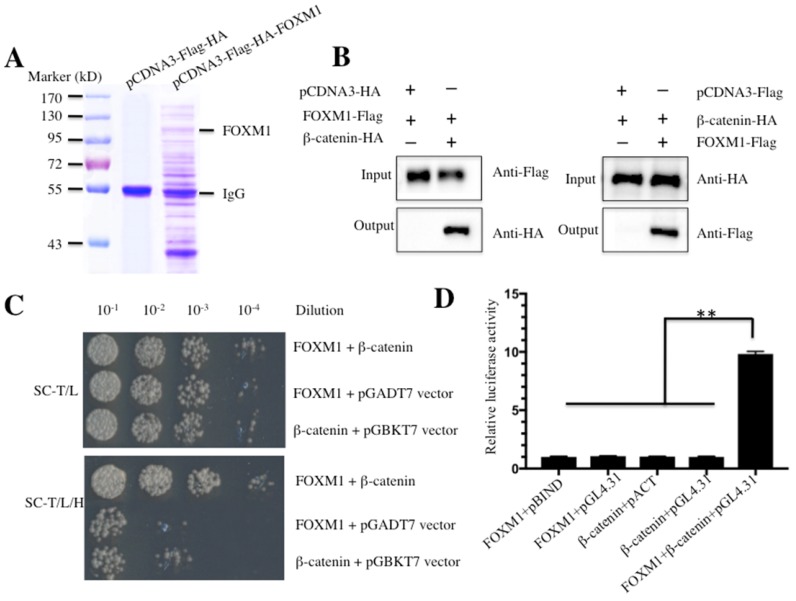
Figure 1.
FOXM1 directly binds to β-Catenin in vitro and in vivo. (A) Immunoprecipitation of the Flag-HA-FOXM1-associated complexes. U2OS cells transfected with pCDNA3-Flag-HA-FOXM1 or pCDNA3-Flag-HA were harvested and subjected to IP analysis by using a two-step approach with anti-Flag-agarose and anti-HA-agarose. The IP protein complexes were subjected to SDS-PAGE separation and staining with Coomassie Brilliant Blue R 250. (B) Co-immunoprecipitation of FOXM1 and β-Catenin in U2OS cells. Cells co-transfected with pCDNA3-FOXM1-Flag and pCDNA3-β-Catenin-HA, or pCDNA3-FOXM1-Flag and pCDNA3-HA (left panel), or pCDNA3-Flag and pCDNA3-β-Catenin-HA (right panel) were co-immunoprecipitated with anti-Flag agarose (left panel) or anti-HA-agarose (right panel). The pull-down products were analyzed via immunoblots with anti-HA and anti-Flag antibodies, respectively. (C) FOXM1 directly bound β-Catenin in yeast. The pGBKT7-FOXM1 plasmid was co-transformed with the plasmid pGADT7-β-Catenin or pGADT7 empty vector into the yeast strain AH109. The resulted yeast colonies were dotted on media minus Trp and Leu (top panel) or minus Trp, Leu, and His (bottom panel) to determine their growth. Serial decimal dilutions were indicated. (D) The interaction of FOXM1 and β-Catenin enhanced the luciferase activity in a M2H assay. Luciferase assay in U2OS cells transfected with pACT-FOXM1 and pBIND, or pACT-FOXM1 and pGL4.31, or pBIND-β-Catenin and pACT, or pBIND-β-Catenin and pGL4.31, or pACT-FOXM1, pBIND-β-Catenin and pGL4.31 were subjected to lucifeare activity assay, which was normalized against the activity of Renilla luciferase. Representative data from three independent experiments are shown. **P<0.001.