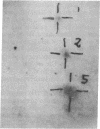
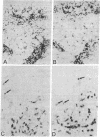
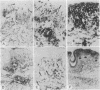
Abstract
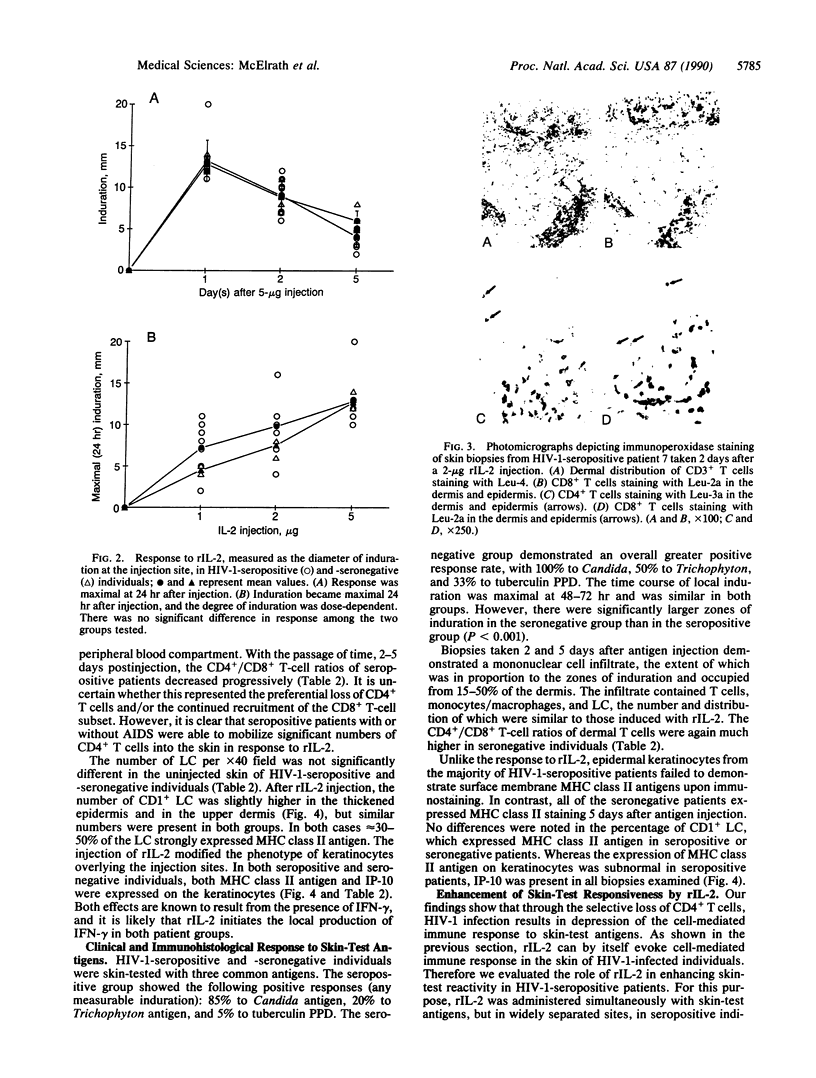
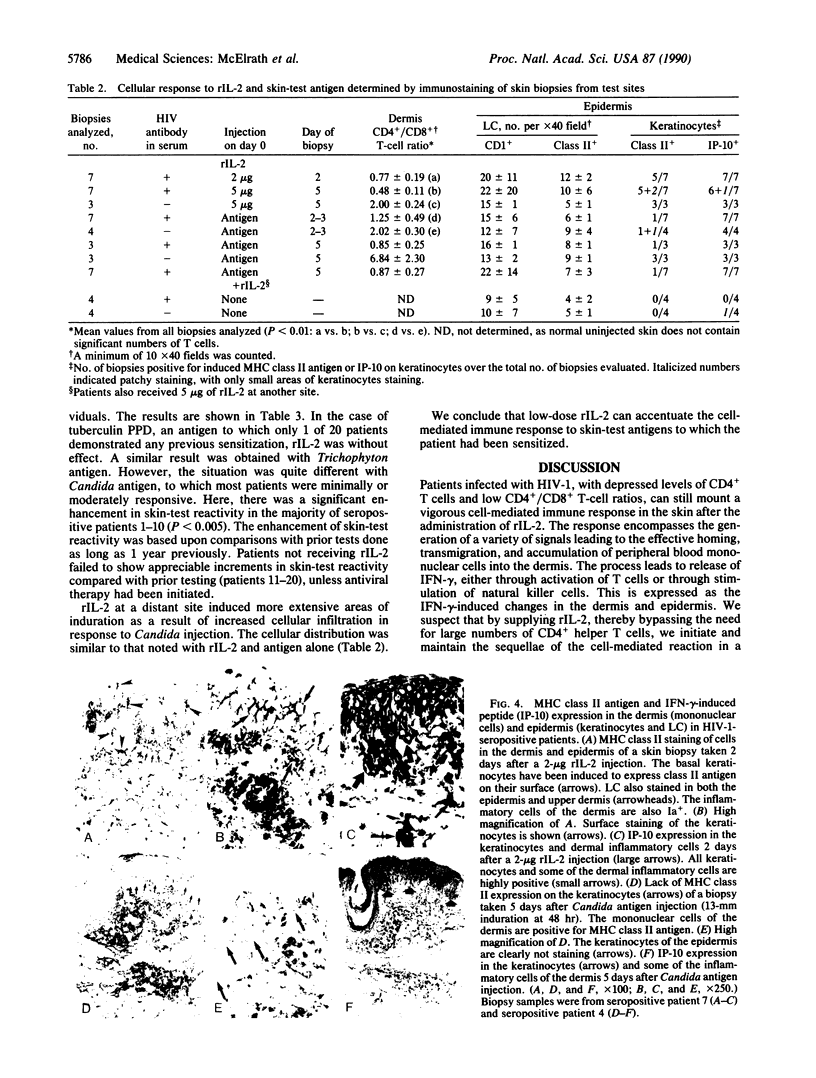
We report that 11 human immunodeficiency virus 1 (HIV-1)-seropositive patients, including three AIDS patients, were able to generate a cellular immune response to the intradermal injection of low doses (2-10 micrograms) of recombinant interleukin 2 (rIL-2). A dose-dependent zone of induration appeared at the site of injection, peaked at 24 hr, and was accompanied by the local accumulation of T cells, monocytes, and Langerhans cells. Despite the reductions in the CD4+ T-cell counts in the peripheral blood of most patients, CD4+ T-cells could still be mobilized with rIL-2 injections into the skin. The total number of immigrant cells was equivalent to those in HIV-1-seronegative patients, although the CD4+/CD8+ ratio of the dermal population was reduced. In response to rIL-2, major histocompatibility complex (MHC) class II antigen was expressed on the surface of keratinocytes, Langerhans cells, lymphocytes, and macrophages. In addition, the gamma interferon (IFN-gamma)-induced protein IP-10 rapidly appeared in dermal inflammatory cells and keratinocytes. A majority of HIV-1-seropositive patients demonstrated low or absent responses to common skin-test antigens. Those with positive zones of induration were often defective in the cellular expression of the IFN-gamma-induced MHC class II antigen. The simultaneous administration of rIL-2 and soluble antigen at widely separated cutaneous sites led to an enhancement of skin-test antigen reactivity in seropositive patients. The results suggest that local administration of rIL-2 to seropositive patients may act systemically, stimulating cellular immunity to recall antigens, and thus may be of potential benefit in the defense against opportunistic pathogens encountered in HIV-1 infection.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bratt G., von Krogh G., Moberg L., Karlsson A., Putkonen P. O., Biberfeld G., Böttiger M., Sandström E. Intradermal testing with multiple recall antigens for identification of cell-mediated immune deficiency in homosexual men. Clin Immunol Immunopathol. 1986 Nov;41(2):206–215. doi: 10.1016/0090-1229(86)90104-2. [DOI] [PubMed] [Google Scholar]
- Gemlo B. T., Palladino M. A., Jr, Jaffe H. S., Espevik T. P., Rayner A. A. Circulating cytokines in patients with metastatic cancer treated with recombinant interleukin 2 and lymphokine-activated killer cells. Cancer Res. 1988 Oct 15;48(20):5864–5867. [PubMed] [Google Scholar]
- Hancock G. E., Cohn Z. A., Kaplan G. The generation of antigen-specific, major histocompatibility complex-restricted cytotoxic T lymphocytes of the CD4+ phenotype. Enhancement by the cutaneous administration of interleukin 2. J Exp Med. 1989 Mar 1;169(3):909–919. doi: 10.1084/jem.169.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan G., Kiessling R., Teklemariam S., Hancock G., Sheftel G., Job C. K., Converse P., Ottenhoff T. H., Becx-Bleumink M., Dietz M. The reconstitution of cell-mediated immunity in the cutaneous lesions of lepromatous leprosy by recombinant interleukin 2. J Exp Med. 1989 Mar 1;169(3):893–907. doi: 10.1084/jem.169.3.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan G., Luster A. D., Hancock G., Cohn Z. A. The expression of a gamma interferon-induced protein (IP-10) in delayed immune responses in human skin. J Exp Med. 1987 Oct 1;166(4):1098–1108. doi: 10.1084/jem.166.4.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan G., Sampaio E. P., Walsh G. P., Burkhardt R. A., Fajardo T. T., Guido L. S., de Miranda Machado A., Cellona R. V., Abalos R. M., Sarno E. N. Influence of Mycobacterium leprae and its soluble products on the cutaneous responsiveness of leprosy patients to antigen and recombinant interleukin 2. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6269–6273. doi: 10.1073/pnas.86.16.6269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane H. C., Depper J. M., Greene W. C., Whalen G., Waldmann T. A., Fauci A. S. Qualitative analysis of immune function in patients with the acquired immunodeficiency syndrome. Evidence for a selective defect in soluble antigen recognition. N Engl J Med. 1985 Jul 11;313(2):79–84. doi: 10.1056/NEJM198507113130204. [DOI] [PubMed] [Google Scholar]
- McElrath M. J., Pruett J. E., Cohn Z. A. Mononuclear phagocytes of blood and bone marrow: comparative roles as viral reservoirs in human immunodeficiency virus type 1 infections. Proc Natl Acad Sci U S A. 1989 Jan;86(2):675–679. doi: 10.1073/pnas.86.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Meuer S. C., Dumann H., Meyer zum Büschenfelde K. H., Köhler H. Low-dose interleukin-2 induces systemic immune responses against HBsAg in immunodeficient non-responders to hepatitis B vaccination. Lancet. 1989 Jan 7;1(8628):15–18. doi: 10.1016/s0140-6736(89)91674-7. [DOI] [PubMed] [Google Scholar]
- Mier J. W., Vachino G., van der Meer J. W., Numerof R. P., Adams S., Cannon J. G., Bernheim H. A., Atkins M. B., Parkinson D. R., Dinarello C. A. Induction of circulating tumor necrosis factor (TNF alpha) as the mechanism for the febrile response to interleukin-2 (IL-2) in cancer patients. J Clin Immunol. 1988 Nov;8(6):426–436. doi: 10.1007/BF00916947. [DOI] [PubMed] [Google Scholar]
- Murray H. W., DePamphilis J., Schooley R. T., Hirsch M. S. Circulating interferon-gamma in AIDS patients treated with interleukin-2. N Engl J Med. 1988 Jun 9;318(23):1538–1539. doi: 10.1056/NEJM198806093182312. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Rubin B. Y., Masur H., Roberts R. B. Impaired production of lymphokines and immune (gamma) interferon in the acquired immunodeficiency syndrome. N Engl J Med. 1984 Apr 5;310(14):883–889. doi: 10.1056/NEJM198404053101404. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Welte K., Jacobs J. L., Rubin B. Y., Mertelsmann R., Roberts R. B. Production of and in vitro response to interleukin 2 in the acquired immunodeficiency syndrome. J Clin Invest. 1985 Nov;76(5):1959–1964. doi: 10.1172/JCI112194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl R. E., Friedman-Kien A., Dubin R., Marmor M., Zolla-Pazner S. Immunologic abnormalities in homosexual men. Relationship to Kaposi's sarcoma. Am J Med. 1982 Aug;73(2):171–178. doi: 10.1016/0002-9343(82)90174-7. [DOI] [PubMed] [Google Scholar]