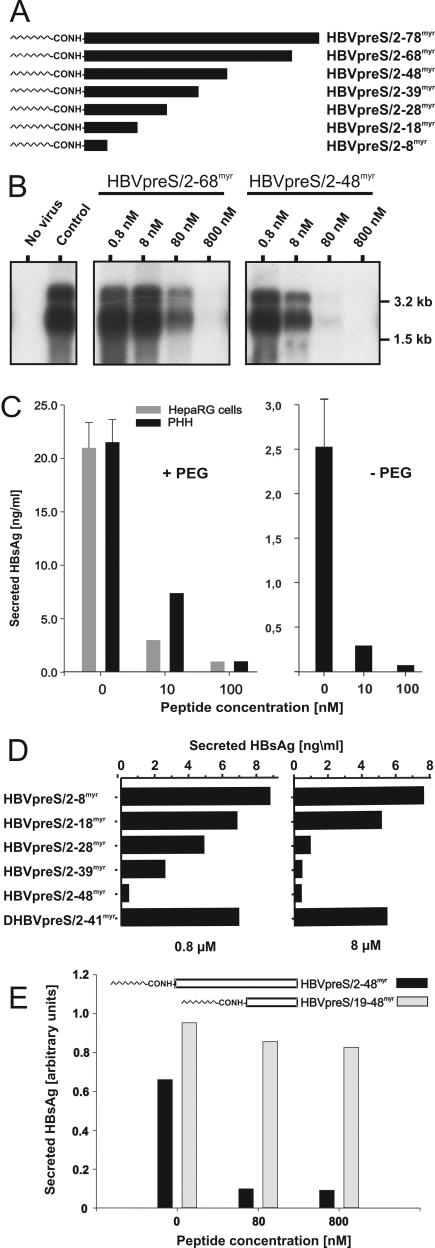
FIG. 1.
Infection inhibition activity of terminally deleted myristoylated HBV pre-S1 peptides. (A) Schematic illustration of HBV L-protein-derived pre-S1 peptides used for infection inhibition. HBVpreS/2-78myr, consisting of the first 77 amino acids of the HBV pre-S1 domain (subtype ayw) as an N-terminal amide of myristic acid (11), and progressively C-terminally deleted myristoylated variants aredepicted on an equivalent scale. Numbers in the names of the peptides specify the first and last amino acids; “myr” indicates that the respective peptide is N-terminally myristoylated at glycine 2. (B) Competition of HBV infection by two myristoylated pre-S1 peptides, HBVpreS/2-68myr and HBVpreS/2-48myr. HepaRG cells were infected with a multiplicity of genome equivalents of ≈50 in the presence of 0.8, 8, 80, and 800 nM concentrations of the respective pre-S peptides. Fourteen hours later, the cells were washed twice with culture medium. At 12 days postinfection, cellular mRNAs were prepared and analyzed by Northern blotting as described previously (11). Both genomic and subgenomic RNAs are represented. The specificity of infection was controlled by an RNA analysis of uninfected HepaRG cells (left lane, no virus); the specificity of inhibition was tested by the addition of the myristoylated DHBV pre-S peptide DHBVpreS/2-41myr at 800 nM (control). RNA size markers are shown on the right. (C) PHH and HepaRG cells were infected overnight under comparable conditions in the presence (left) or absence (right) of PEG, with 0, 10, or 100 nM HBVpreS2/48myr as an inhibitor. After removal of the peptide, the medium was exchanged every 2 to 3 days and the amount of HBsAg was determined from days 12 to 14 postinfection. Error bars in the uncompeted infections represent the standard deviations for six independent infections. (D) HepaRG cells were infected in the presence of 0.8 μM (left) and 8 μM (right) HBVpreS/2-8myr, HBVpreS/2-18myr, HBVpreS/2-28myr, HBVpreS/2-39myr, and as controls, HBVpreS/2-48myr and DHBVpreS/2-41myr. The infectious inoculum and the peptides were incubated overnight, washed, and replaced with new medium. At 12 days postinfection, the collected cell culture supernatants from days 8 to 12 were analyzed for secreted HBsAg by use of a quantitative commercially available ELISA. The results obtained are given in nanograms of HBsAg per milliliter of cell culture medium. (E) Comparative HBV infection assay with the two myristoylated pre-S1-derived peptides HBVpreS19-48myr, which lacks the first 17 pre-S1 amino acids and carries an artificial myristic acid residue at the N-terminal leucine 19, and HBVpreS/2-48myr. HepaRG cells were infected with HBV (MGE = 50) for 12 h in the presence of 8, 80, and 800 nM concentrations of the respective peptide. The medium was changed, and newly synthesized HBsAg was determined between days 8 and 12 postinfection. Values are given as relative units (optical densities) obtained from the ELISA reader.