Figure 2.
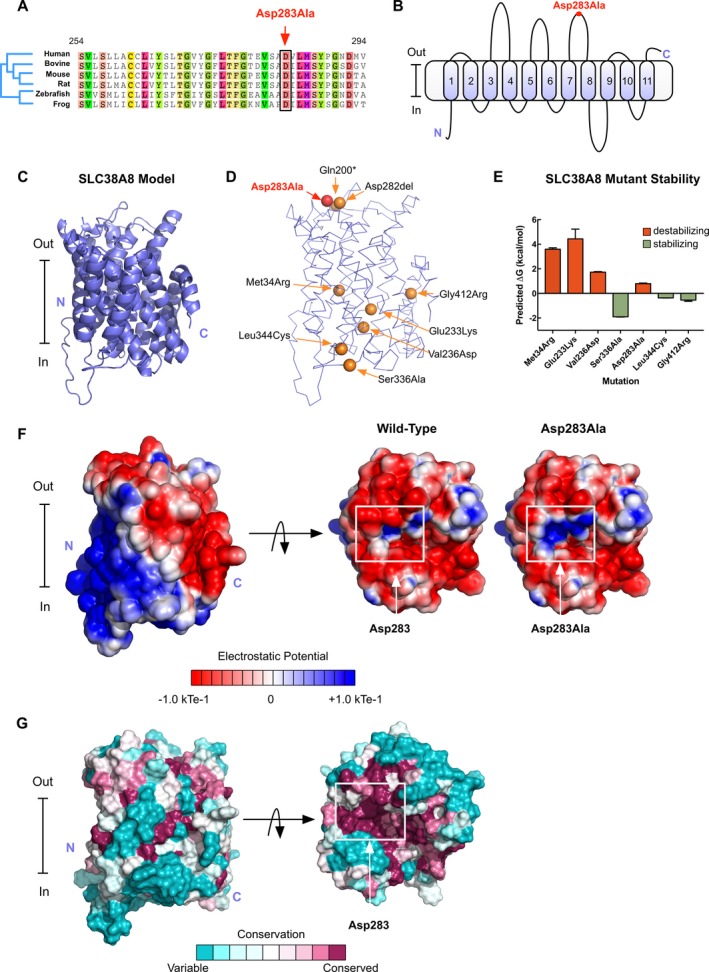
Structural modeling of patient SCL38A8 mutations: (A) Sequence alignment showing the p.Asp283 position to be conserved across multiple species. (B) Membrane topological structure diagram predicts SLC38A8 to have 11 transmembrane helices and places the p.Asp283Ala mutation on the extracellular surface of the channel. (C) Our SLC38A8 model generated using the I‐TASSER program. (D) Known FHONDA‐associated mutations mapped onto our SLC38A8 model. (E) Mutations were introduced into our model using FoldX and predicted changes in total energy were calculated. Positive changes in total energy were predicted to be destabilizing. (F) Electrostatic potential surface of our SLC38A8 model shows a more positive potential surface at the site of the mutation near the opening of the channel pore. (G) Consurf coloring of the SLC38A8 model reveals the p.Asp283 residue to be 100% conserved across 103 homologous sequences.
