Abstract
Objective
Hospital services use, which is a major driver of total health expenditures, is expected to rise over the next decades in Europe, especially because of population aging. The purpose of this article is to better understand the dynamics of older people's demand for hospital care over time in a cross‐country setting.
Data source
We used data from the Survey on Health, Ageing, and Retirement in Europe (SHARE), in 10 countries between 2004 and 2011.
Study Design
We estimated a dynamic panel model of hospital admission for respondents aged 50 years or more.
Principal Findings
Following prior research, we found evidence of state dependence in hospital use over time. We also found that rise in frailty—among other health covariates—is a strong predictor of increased hospital use. Progression by one point on the frailty scale [0;5] is associated with an additional risk of about 2.1 percent on average.
Conclusions
Our results support promotion of early detection of frailty in primary care, and improvement of coordination between actors within the health system, as potential strategies to reduce avoidable or unnecessary hospital use among frail elderly.
Keywords: Demand for health, long‐term care, frailty, health prevention, dynamic panel
Hospital services use, which is a major driver of total health expenditures, is expected to rise over the next decades in Europe, especially because of population aging (Kohn and Liu 2013; Ilinca and Calciolari 2015). Hospitals services, which are traditionally the major source of long‐term care expenditure (Forder 2009), have been at the core of many recent measures aimed at cutting costs and gaining efficiency. A central aspect is in the management of disease by primary care producers, which can lead to large savings due to prevented hospitalizations (Dusheiko et al. 2011). In that context, most European countries are refocusing health policy on health promotion and disability prevention among older people. From an economic perspective, reducing nonplanned hospitalization can lead to large savings. Previous research provided evidence that almost 5 percent of the hospitals' production costs were dedicated to the holding of reserve capacity to service this stochastic demand (Hughes and McGuire 2003). The purpose of this article is to better understand the drivers of older people's demand for hospital care over time in a cross‐country setting.
Modeling individuals' demand for hospital care in the elderly population is a complex process. Three key aspects must be taken into consideration. First, hospitalization should be modeled as a dynamic process. In Britain, previous research provided evidence that past hospitalization was associated with lower future length of stays in individuals' hospital use (Kohn and Liu 2013). That hospitalization use should be modeled as a dynamic process was confirmed in Norway, were hospital readmission was lower for patients experiencing longer length of stays (Bjorvatn 2013). Second, models should control for patterns of primary care providers' visits, as they play a central role in such hospital use dynamics. The quality of the care produced by general practitioners (GPs) in Norway was a key predictor of hospital use (Carlsen et al. 2007). Financial incentive schemes provided to GPs in Italy were associated with lower rates of preventable hospital admissions (Fiorentini et al. 2011). Third, health covariates should include measures of frailty to get a more accurate assessment of older people's need for care. Frailty is found to be a strong predictor of higher older people's hospital use over time (McAdams‐DeMarco et al. 2013; Ilinca and Calciolari 2015).
Our original contribution is to combine these three key aspects to develop a comprehensive model of older people's hospitalization use over time. In the detail, we estimated a dynamic panel model of hospital admission for more than 7,500 respondents of the Survey on Health, Ageing, and Retirement in Europe (SHARE) in 10 countries between 2004 and 2011. Using the reference methodology defined by Kohn and Liu (2013), we specified a dynamic model for hospital use.
Special attention is given to the assumption of state‐dependency of medical health care to explain the dynamics of hospital admissions. We explore the role of visits to GPs in the explanation of the hospital use dynamic. We also explore to what extent hospital care is a substitute to specialist practitioners (SPs) service. We control for health by using a set of covariates, including a new measure of frailty (Fried et al. 2001, 2004; Lang, Michel, and Zekry 2009) since frailty is a transitional state from healthy aging to functional decline.
Data
Source and Sample
SHARE is a multidisciplinary and cross‐national cohort of individual data on health, socio‐economic status and social and family relationships of more than 80,000 respondents aged 50 years or older (Borsch‐Supan et al. 2013). Eleven countries contributed to the 2004 SHARE baseline study (Israel took also part in SHARE wave 1 only). They are a balanced representation of the various regions in Europe, ranging from Scandinavia (Denmark and Sweden) through Central Europe (Austria, France, Germany, Switzerland, Belgium, and the Netherlands) to the Mediterranean (Spain, Italy, and Greece). Further data were collected in 2006–2007 during the second wave of SHARE in these countries, the Czech Republic, Poland, and Ireland. SHARELIFE, the third wave of the project, was conducted in 2008–2009 over the same population (apart from Ireland). This time, the respondents were interviewed about their life history. Different fields such as childhood health, education, job career, family life, housing, and so on were surveyed and provide useful information on initial conditions and life course. In 2010, Greece dropped from the survey (as a consequence of the economic crisis) while Estonia, Slovenia, Hungary, and Portugal joined SHARE wave 4, which is the third regular panel wave of the survey following the SHARELIFE life history questionnaire.
The sample retained here is balanced; it is made of the three regular panel waves and a retrospective one. It is restricted to the 10 baseline countries, which did carry all of the four waves in northern (Denmark, Sweden, the Netherlands), continental (Austria, Germany, France, Belgium, Switzerland), and southern (Italy, Spain) regions of Europe. Finally, only full‐rank data matrices are kept at each wave so that respondents with missing data are deleted. Our final sample consisted of 30,248 observations made of 7,562 individuals repeatedly surveyed each time during the first four waves of SHARE.
Description of the Variables
Our dependent variable is a dichotomous variable measuring whether, during the last 12 months, the respondent has been in a hospital overnight. Our explanatory variables of interest measured whether the respondent has seen or talked to a GP about his/her health and has consulted any of the SP mentioned on a list. We also created three lag variables measuring the use of GPs, SPs or hospital during the previous wave. Following prior work (Santos‐Eggimann et al. 2009), we derived Fried's frailty measure based on five criteria (exhaustion, shrinking, weakness, slowness, and low activity) corresponding to various subjective and objective health measures in SHARE. The initial score of frailty takes its values in the interval [0;5] since one point was allocated for each fulfilled criterion.
In addition to these variables of interest, we controlled for several indicators of health status to take into account the need for care. A dichotomous variable was created for each of the following health measure: self‐rated health (SRH) being fair or poor; multimorbidity for at least two chronic diseases; two or more functional limitations Katz's activities of daily living (ADLs) and Lawton's instrumental activities of daily living (IADLs); and the Euro‐D measure for depressive symptoms (Prince et al. 1999). A composite health index was derived from a multiple correspondence analysis of the previous health measures and a dichotomous variable is created for general “poor health.” The multiple correspondence analysis (Greenacre 2006) results indicate that the first component captures most of the information in the binary health variables. Individual coordinates of the first axis thus provide a synthetic health index made of a nonlinear combination of the binary health variables. Since positive values and negative values of this index reflect general poor or good health, a binary index was derived for the sake of simplicity. The health index is made of the individuals' coordinates on the first axis as a data reduction approach. We substituted this health index for all health variables but frailty in another model to test for change in standard errors due to potential multicolinearity as there is a wide array of health measures in the covariates. As the results were identical, we are confident in our estimates with full health measure, which gives the reader a better appraisal on the economic importance of each health condition on hospital stay. This measure provided a good summary of trends in health over the period. Additional covariates include a measure of income adequacy on a 4‐point scale, whether the household is able to make ends meet: 1‐with great difficulty, 2‐with some difficulty, 3‐fairly easily, or 4‐easily. Income adequacy is the only measure of economic well‐being that is consistent over the different waves of SHARE—as measures of income and assets changed between waves 1 and 2. Finally, we control for a variable measuring the presence of health problems in adult life. This variable is taken from SHARELIFE, the retrospective survey that took place in wave 3. Respondents were asked about their life history. “Health problems in adult life” is a binary index of health, taking the value 1 if the respondent reports any periods of ill health over the life‐cycle (>1 year) or if she reports any physical injury over the lifecycle (>1 year). As health problems and hospital use in late life is found to be associated with health factors in early‐ and mid‐life (Freedman et al. 2008), this variable is used as a proxy for hospital use before the start of the prospective panel.
Methods
We used dynamic panel models for binary outcome. The simple estimation procedure suggested by Wooldridge contributed to render these methods increasingly popular in applied economics (Wooldridge 2005). We followed a seminal application to health economics that was provided in previous research (Contoyannis, Jones, and Rice 2004) and recently applied to hospital care consumption (Kohn and Liu 2013). Following that model, we used a dynamic specification by including an autoregressive one‐period lag of the hospital use variable to reflect persistence, or state dependence.
Let y it denote the binary outcome of hospital stays for individual i, i = 1, … , N, at time t, t = 1, …,T. Let X it denote the full‐rank data matrix of explanatory variables. We assumed additive unobserved heterogeneity in a dynamic probit model such as:
| (1) |
where c i is the unobserved individual specific term and Φ is the normal cumulative distribution function. Unlike in the static case, dynamic panel models typically rest on sequential moments restrictions (Chamberlain 1992). However, the usual random effect assumption of strict independence between c i and X it is too strong in the case of individual micro‐data. We therefore allowed some correlation between the unobservable and the explanatory variables in order to retain a more realistic assumption. Following previous research (Ilinca and Calciolari 2015), we applied a Mundlak‐Chamberlain device where the individual fixed effect is netted out using a constant and a time‐trend:
| (2) |
where c i, the unobserved individual effect is replaced by its linear projection onto , the means of the regressors, and where ψ is the intercept, y i0 is the initial value of y it prior to the start of the survey, and a i represents the projection error. Our assumption is an application of the Mundlak‐Chamberlain device where the individual fixed effect is netted out using a constant and a time‐trend. An advantage of this methodology is that our model estimates are not subject to omitted variable bias as long as the omitted variables are time‐invariant. In other words, controlling for gender, age, education, being a migrant, or other individual fixed effects would not modify the parameters of the time‐varying variables. Similarly, average partial effects for the explanatory variables can now be interpreted as within estimates, just like an alleged “fixed effects probit” model would yield.
The use of y i0 prevents inconsistent estimates—known as the problem of initial conditions (Heckman 1981). Although it is general practice to use the first wave observation as the initial value of the dependent variable, life‐history data from wave 3 (SHARELIFE) allow us to use retrospective information as a good proxy for y i0. Doing so, we kept the first regular panel wave for the analysis so that T = 3 is sufficient for a dynamic estimation. Indeed, panel data analysis requires at least two periods to analyze at least one transition, if the panel model includes a lagged variable, then another time period is needed. We opted for the variable health problems in adult life, a binary retrospective index of health taking the value 1 if the respondent reported any periods of ill health over the life‐cycle (>1 year) or if she reported any physical injury over the lifecycle (>1 year).
Lee (2005) points out that dynamic panel models usually rely on the implicit assumption that one lag of the dependent variable is sufficient to fully capture the dynamics of the process (Lee 2005). Past hospital use may be associated with other health care use in the preceding period, especially specialist care since in some European countries, specialist care can only be found at the hospital. Put differently, it may also be that visit to GPs and SPs at t − 1 would be associated with hospital visit at t − 1, either as complement or substitute care to hospital visit (Carlsen et al. 2007; Ilinca and Calciolari 2015). As a consequence, visit to GPs and SPs at t − 1 are included as control variables besides hospital visits at t − 1. In addition, visit to GPs and SPs at t are also included as determinants of hospital visits at time t. Other covariates include health measures, the variable of make‐ends‐meet, time dummies (to correct for the time‐spell between regular panel waves 1, 2, and 4), and a constant.
We used simple structural coefficient tests to define our model specification. These tests supported the dynamic approach, failed to reject the Mundlak‐Chamberlain device, and failed to reject the influence of initial conditions, meaning that a genuinely new process is observed at the beginning of the sample. We can easily extend the specification of (1) to achieve dynamic completeness; under assumption (2), this defines a latent variable regression model:
| (3.1) |
| (3.2) |
| (3.3) |
where Z it contains health measures, the variable of make‐ends‐meet, time dummies (to correct for the time‐spell between regular panel waves 1, 2, and 4), and the constant ψ. We also assume that and ɛ it ∼ N(0, 1). Practical estimation of δ, β, ξ, ξ 0, and σ 2 a is straightforward using statistical routines for random effect probit models (e.g., ‐xtprobit‐ in Stata). In the detail, matrix δ contains δ y, δ gp, δ sp the coefficients of the respective lagged values of hospital visits, GP visits, and SP visits. Matrix β contains β gp, β sp, β z the coefficients of the respective values of GP visits, SP visits, and other covariates in Zit which are: health measures, the make‐ends‐meet variable, the wave dummies, and the constant. ξ is the matrix of coefficients associated with averaged values over time (Mundlak parameters) of GP visits, SP visits, health measures, and the make‐ends‐meet variable. ξ 0 is the parameter for the “health problems in adult life.”
Simple structural coefficient tests provide useful guidance for model specification. First, H0: ρ = 0 favors the static model, while HA: ρ ≠ 0 indicates state dependence and supports the dynamic approach. Second, H0: ξ = 0 can be interpreted as a pseudo‐Hausman test for random effects since H0 indicates absence of correlation between c i and Z it, which clearly rejects the Mundlak‐Chamberlain device in favor of the baseline random effects probit. Third, H0: ξ 0 = 0 rejects the influence of initial conditions, meaning that a genuinely new process is observed at the beginning of the sample.
We estimated two different models to explore two main research questions. First, we explored the determinants of hospital stays. We ran a static version of the probit model for panel data with the Mundlak‐Chamberlain device (M1), a dynamic version with initial conditions and the lagged dependant variable, and with addition of lags of ambulatory care variables (M2). Note that average partial effects (APEs) are presented instead of coefficients for the sake of comparability across models.
The validity of the dynamic panel model estimated on a balanced sample rests on the particular assumption that sample attrition is exogenous, meaning that results derived from a specific sample are independent to the reasons why some respondents dropped out of the survey. Under this assumption, estimates from the balanced and unbalanced samples should not be different. The three waves of regular panel in SHARE are reduced to two repeated observation points when one lag period is considered. As a consequence, the dynamic model cannot be estimated on an unbalanced sample, and the test procedure for exogenous attrition can only be based on the static model M1. In practice, balanced sample observations were duplicated within the working sample and the set of contemporaneous regressors is duplicated for balanced and unbalances samples. A dummy indicating whether the observation has been duplicated was added as a control variable and the model was estimated over the whole sample. Notice that time dummies were also included among the regressors. Inference on APEs between balanced and unbalanced subsamples is straightforward within this single model specification.
Our attrition test is based on a version of a Hausman test for nonresponse suggested by Nijman and Verbeek (1992). Although the authors propose the test for nondynamic models, it is standard practice to use it in a dynamic framework (e.g., Contoyannis, Jones, and Rice 2004). There are two reasons for that. First, attrition test on a nondynamic panel model is less subject to bias, as it relies on a minimum number of two periods whereas a dynamic test makes use of at least three periods (two for the panel structure and one for the lag of hospital use). Second, if attrition is endogenous, that is, if it is related to our dependent variable, then a lag of hospital use takes into account some of the reasons why attrition occurs (or attrition on observables). Fitzgerald et al. (1998) proposed a test for “dynamic attrition” and discuss its relative merit in the case for selection on unobservables.
Results
Descriptive Statistics
Figure 1 displays the value of the measures of health care use and health status for the same individuals over the four waves of SHARE. These measures are only available for the regular panel waves since wave 3 (SHARELIFE) was dedicated to life‐history data collection. The general upward trend indicates both need for care and health care consumption increase as individuals are aging. The left‐hand side graph in Figure 1 presents the distribution of the types of care used, with a wide access to GP (on average, 83 percent of the sample went to visit a GP between wave 1 and 4), and a more restricted consumption of specialist care (46.2 percent of the sample) and hospital stays (13.2 percent). Notice that the rapid surge in specialist care use (3.1 percentage points per wave) is found to be associated with the fast evolution of multimorbidity or other health measures. First difference estimates (conditional logit) indicate that after controlling for individual time‐invariant characteristics, time trends, and the usual time‐varying characteristics (financial situation, use of other health care resources: GP and hospital stays), health measures (a synthetic health index and the frailty phenotype) are strongly associated with visits to SP (p < 1 percent).
Figure 1.
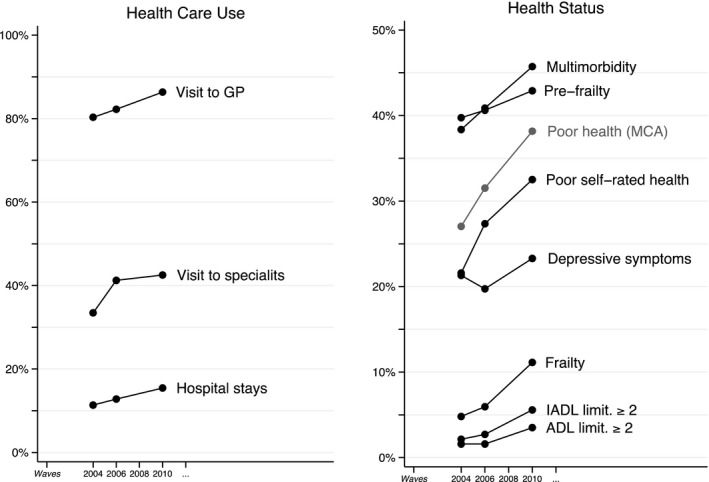
Prevalence and Incidence of Health Status and Health Care Use in Europe
Table 1 provides an overview of the average values of the variables at different points in time. Not surprisingly, care use (hospital, SP, and GP visits) is increasing in time, along with the levels of functional limitations (ADL, IADL, and frailty), depressive symptoms, and chronic conditions. Financial resources are stable over the study period.
Table 1.
Sample Description—Average Values
| Variables | All Countries | |||||||
|---|---|---|---|---|---|---|---|---|
| Wave 1 | Wave 2 | Wave 4 | Overall | |||||
| Obs. (1) | Missing (2) | Obs. (1) | Missing (2) | Obs. (1) | Missing (2) | Obs. (1) | Missing (2) | |
| Health care use | ||||||||
| Hospital stays | 0.114 | 0.001 | 0.128 | 0.001 | 0.154 | 0.004 | 0.132 | 0.002 |
| Visit to GP | 0.803 | 0.003 | 0.822 | 0.005 | 0.864 | 0.009 | 0.830 | 0.006 |
| Visit to SP | 0.334 | 0.003 | 0.412 | 0.005 | 0.425 | 0.009 | 0.391 | 0.006 |
| Need for care | ||||||||
| Fried's Frailty index [0;5] | 0.662 | 0.049 | 0.718 | 0.069 | 0.947 | 0.098 | 0.776 | 0.072 |
| Poor SRH | 0.216 | 0.001 | 0.273 | 0.001 | 0.325 | 0.002 | 0.271 | 0.001 |
| Chronic 2+ | 0.384 | 0.001 | 0.409 | 0.001 | 0.457 | 0.002 | 0.416 | 0.001 |
| Limit. w/IADL 2+ | 0.021 | 0.001 | 0.027 | 0.001 | 0.056 | 0.002 | 0.035 | 0.001 |
| Limit. w/ADL 2+ | 0.016 | 0.001 | 0.016 | 0.001 | 0.035 | 0.002 | 0.022 | 0.001 |
| Depressive sympt. | 0.213 | 0.010 | 0.197 | 0.013 | 0.233 | 0.028 | 0.214 | 0.017 |
| Resources | ||||||||
| Make‐ends‐meet | 2.973 | 0.009 | 3.015 | 0.019 | 3.104 | 0.021 | 3.031 | 0.016 |
| Initial conditions | ||||||||
| Health problems in adult life | 0.261 | 0.003 | 0.261 | 0.003 | 0.261 | 0.003 | 0.261 | 0.003 |
| Other indiv. char. | ||||||||
| Female | 0.536 | 0.000 | 0.536 | 0.000 | 0.536 | 0.000 | 0.536 | 0.000 |
| Years of age | 63.065 | 0.000 | 65.364 | 0.000 | 69.649 | 0.000 | 66.026 | 0.000 |
| Overall missing rate | 0.066 | 0.091 | 0.118 | 0.091 | ||||
(1) N = 7,562 (2) N = 9,444 individuals answer the survey at waves 1, 2, and 4. However, some respondents do not answer all the questions. Only 7,562 individuals answer all questions at all waves. As a consequence, the overall individual attrition rate is 20% (7,562/9,444) since missing observations at any wave lead to attrition in a balanced sample.
Conditional Evolution of Hospital Rates
Table 2 presents the APEs of the determinants of hospital stays. Our static model (M1) estimates suggest that increase in hospital stays between waves is essentially associated with increase in the need for care; as most health measures are significant, apart from limitations with IADL (>10 percent) or ADL (<10 percent). Progression from “good health” toward poor SRH or multimorbidity increases the risk of occurrence of hospital stays by 5 and 3 percent, respectively. The presence of more than three depressive symptoms is associated with some 1.6 percentage point increase. Progression by one point on the frailty scale [0;5] is associated with an additional risk of about 2.1 percent on average. Consumption of alternative source of care is associated with hospital stays: APEs for visits to GP and SP are of 6.5 and 11.8 percent, respectively. Finally, the Pseudo‐Hausman tests for the Mundlak‐Chamberlain device in M1 rejects the random effect assumption (χ2 = 21.06; p < 1 percent), suggesting that the interpretation of the results should follow from a hypothetical “fixed effects probit model.”
Table 2.
Average Partial Effects (APEs) of the Determinants of Hospital Stays
| Dep var: Hospital Stays (t) | Static Model | Dynamic Model | ||
|---|---|---|---|---|
| Explanatory Variables | APE | SE | APE | SE |
| Past health care use | ||||
| Hospital stays (t − 1) | 0.052*** | 0.007 | ||
| Visit to GP (t − 1) | −0.002 | 0.013 | ||
| Visit to SP (t − 1) | −0.024*** | 0.009 | ||
| Contemporaneous altern. care | ||||
| Visit to GP | 0.065*** | 0.009 | 0.075*** | 0.014 |
| Visit to SP | 0.118*** | 0.006 | 0.121*** | 0.009 |
| Contemporaneous need for care | ||||
| Frailty index [0;5] | 0.021*** | 0.004 | 0.025*** | 0.005 |
| Poor SRH | 0.049*** | 0.007 | 0.077*** | 0.009 |
| Chronic 2+ | 0.031*** | 0.007 | 0.025*** | 0.009 |
| Limit. w/IADL 2+ | 0.029* | 0.015 | 0.036* | 0.019 |
| Limit. w/ADL 2+ | 0.011 | 0.017 | 0.027 | 0.022 |
| Depressive sympt. | 0.016** | 0.008 | 0.021** | 0.010 |
| Contemporaneous resources | ||||
| Make‐ends‐meet | −0.006 | 0.004 | −0.009 | 0.005 |
| Initial conditions | ||||
| Health problems in adult life | 0.020*** | 0.006 | ||
| Time fixed effects | ||||
| Wave 1 | Ref. | Ref. | ||
| Wave 2 | −0.004 | 0.005 | Ref. | Ref. |
| Wave 4 | 0.006 | 0.005 | 0.009 | 0.006 |
| (+ Mundlak device) | ||||
| Tests on APEs (χ2, p‐value) | ||||
| Pseudo‐Hausmann test | 21.1 | 0.012 | 35.9 | 0.000 |
| H0: βGPt = βSPt | 476.3 | 0.000 | 238.2 | 0.000 |
| H0: δGPt‐1 = δSPt‐1 | 7.4 | 0.024 | ||
| Obs. | 22,686 | 15,124 | ||
The static model explores the association between current care use variables and hospital use. The dynamic model explores the association between care use at the previous wave and current hospitalizations.
*p < .1, **p < .05, ***p < .01.
The dynamic model (M2) provides three main additional results. First, we find evidence of persistence in the dynamics of hospital stays (APE = 5 percent; p < 1 percent). Second, the static model (M1) fit can be improved by inserting lagged variables. Note that the significant influence of initial conditions in M2 increases confidence in the dynamic specification of the model as presample individual characteristics influence the dynamics of y it. Third, M2 also indicates that although model specification should be dynamic, a lagged dependent variable is not enough to capture the full dynamic of hospital stays. We find that the presence of previous visits to specialists contributes to reduce the probability of hospitalization.
Testing for Exogenous Attrition
Table 3 presents the results of the attrition test. Notice that APEs from the same estimated equation are displayed in the column so that equality tests can be displayed on the same row. Our results indicate that each explanatory variable has statistically the same APE in both subsamples (all p‐values >10 percent). A joint test is also carried out (Wald t‐test = −1.08; p = .280) and concurs with previous individual tests that attrition in the working sample is exogenous. In addition, the APE associated with the sample dummy is not significant and indicates that conditional rates of hospital stays do not differ between subsamples. By and large, these tests suggest that attrition in the working sample does not modify the determinants of the hospital care demand function.
Table 3.
Exogenous Attrition Tests
| Dep var: Hospital Stays | Average Partial Effects | Test of APEs Equality | ||||||
|---|---|---|---|---|---|---|---|---|
| Explanatory Variables | Selected Sample | Full Sample | Diff. | SE of Diff. | Wald t‐test | p‐value | 95% CI | |
| Health care use | ||||||||
| Visit to GP | 0.051*** | 0.057*** | −0.006 | 0.007 | −0.89 | 0.374 | −0.020 | 0.008 |
| Visit to SP | 0.091*** | 0.092*** | −0.002 | 0.004 | −0.35 | 0.727 | −0.010 | 0.007 |
| Need for care | ||||||||
| Frailty index [0;5] | 0.020*** | 0.019*** | 0.001 | 0.002 | 0.58 | 0.563 | −0.003 | 0.006 |
| Poor SRH | 0.041*** | 0.042*** | −0.001 | 0.005 | −0.19 | 0.852 | −0.011 | 0.009 |
| Multimorbidity | 0.025*** | 0.025*** | 0.000 | 0.005 | −0.06 | 0.954 | −0.009 | 0.009 |
| Limit. w/IADL 2+ | 0.016** | 0.020** | −0.005 | 0.010 | −0.45 | 0.653 | −0.025 | 0.016 |
| Limit. w/ADL 2+ | 0.006 | 0.009 | −0.003 | 0.012 | −0.26 | 0.799 | −0.027 | 0.021 |
| Depressive sympt. | 0.012*** | 0.015*** | −0.002 | 0.005 | −0.44 | 0.657 | −0.013 | 0.008 |
| Resources | ||||||||
| Make‐ends‐meet | −0.001 | −0.002 | 0.001 | 0.002 | 0.40 | 0.691 | −0.004 | 0.006 |
| Joint model controls | ||||||||
| Sample dummy | 0.004 | |||||||
| (+ Wave dummies) | ||||||||
| (+ Mundlak device) | ||||||||
| Joint test | −0.017 | 0.016 | −1.08 | 0.280 | −0.048 | 0.014 | ||
| Obs. | 94,769 | |||||||
| Obs. by subsample | 22,686 | 72,083 | ||||||
*p < .1; **p < .05; ***p < .01.
Conclusion
This study provides new evidence on the determinants of hospital admission rates in the general population over the recent years in Europe. We added two main contributions to the literature. First, we confirmed previous findings between healthy aging and hospitalization using a measure of frailty in the general population (Ilinca and Calciolari 2015). Second, we enhanced that model by combining the various approaches found in the literature to develop a comprehensive model that explored the dynamics of care use (Kohn and Liu 2013). We found that previous SP visits prevent future hospitalizations. This last result is of prior importance since it suggests a potential substitution effect between hospital care and specialist care. Indeed, outpatients' appointments are counted as SP visits: ambulatory specialists' visits are likely to substitute for hospital care, explaining the negative association with admissions.
Our results are consistent with previous research. Kohn and Liu (2013) have found similar persistence in the dynamics of health care use using British data. This confirmed the choice of a dynamic panel model specification. Ilinca and Calciolari (2015) have found similar association between frailty and hospitalization risks. This autonomous effect of frailty confirms that a share of the population with increasing risks of incapacity remains overlooked by means of the usual markers for incapacity, although these individuals have a higher propensity to experience hospital stays. In other words, the frailty index is an interesting potential candidate for screening subpopulations at risk who would usually fall out of the scope of prevention.
Our results support two main strategies that can be observed among European countries. The first one is essentially to promote early detection of frailty at the level of general practice to foster differential treatment in primary care. For instance, in France, a Platform for Evaluation of Frailty and Prevention of Disability has been created to identify the early signs of frailty and to design more effective interventions (Subra et al. 2012). The second strategy consists in improving coordination between actors within the health system (or integrated care), and—what is yet much less of a priority of on‐going reforms—to crucially tackle significant health inequalities in access to specialist care among the elderly. For instance, in the Netherlands, the Prevention and Reactivation Care Program and the Recovery Care Program represent personalized, integrated interventions for the prevention of functional decline after hospital stay, and the improvement of functioning for vulnerable elderly people.
Our results have important health policy implications, as they suggest that offering integrated solutions to frail elderly people would result in overall reduced hospital rates. This article brings support to the implementation of integrated solutions to prevent elderly people's hospitalizations. As frailty usually precedes dependence, health care planners show an increasing interest in developing interventions designed to prevent expensive care trajectories among frail people (De Lepeleire et al. 2009). There has been evidence that such interventions reduced the length of hospitalizations by more than 50 percent, leading to important cost savings (Brunenberg et al. 2005). Consequently, the assumption that targeting frail adults could slow down the growth of health care spending among the elderly, mainly through a reduction of hospitalization rates, is appealing and has gained wide acceptance among policy makers. The emphasis is put on the role of GPs to identify frail older people to provide early intervention and/or multidisciplinary case management (De Lepeleire et al. 2009). Further research should explore the cost‐effectiveness of interventions designed to improve frailty detection in primary care.
Supporting information
Appendix SA1: Author Matrix.
Table A1: Descriptive Statistics by Country and Waves (Average Values).
Acknowledgments
Joint Acknowledgment/Disclosure Statement: The research leading to these results has received support from the SPRINTT project of the Innovative Medicines Initiative Joint Undertaking under grant Agreement number 115,621, resources of which are composed of financial contribution from the European Union's Seventh Framework Program (FP7/2007–2013) and EFPIA companies' “in kind contribution.” This article uses data from SHARE wave 4 release 1.1.1, as of March 28, 2013 (DOI: 10.6103/SHARE.w4.111) or SHARE wave 1 and 2 release 2.6.0, as of November 29, 2013 (DOI: 10.6103/SHARE.w1.260 and 10.6103/SHARE.w2.260) or SHARELIFE release 1, as of November 24, 2010 (DOI: 10.6103/SHARE.w3.100). The SHARE data collection has been primarily funded by the European Commission through the 5th Framework Program (project QLK6‐CT‐2001‐00360 in the thematic program Quality of Life), through the 6th Framework Program (projects SHARE‐I3, RII‐CT‐2006‐062193, COMPARE, CIT5‐CT‐2005‐028857, and SHARELIFE, CIT4‐CT‐2006‐028812) and through the 7th Framework Program (SHARE‐PREP, NO. 211909, SHARE‐LEAP, No. 227822 and SHARE M4, No. 261982). Additional funding from the U.S. National Institute on Aging (U01 AG09740‐13S2, P01 AG005842, P01 AG08291, P30 AG12815, R21 AG025169, Y1‐AG‐4553‐01, IAG BSR06‐11, and OGHA 04‐064) and the German Ministry of Education and Research as well as from various national sources is gratefully acknowledged (see www.share-project.org for a full list of funding institutions).
Disclosures: None.
Disclaimers: None.
References
- Bjorvatn, A. 2013. “Hospital Readmission among Elderly Patients.” European Journal of Health Economics: HEPAC: Health Economics in Prevention and Care 14: 809–20. [DOI] [PubMed] [Google Scholar]
- Borsch‐Supan, A. , Brandt M., Hunkler C., Kneip T., Korbmacher J., Malter F., Schaan B., Stuck S., Zuber S., and Team S. C. C.. 2013. “Data Resource Profile: The Survey of Health, Ageing and Retirement in Europe (SHARE).” International Journal of Epidemiology 42: 992–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunenberg, D. E. , van Steyn M. J., Sluimer J. C., Bekebrede L. L., Bulstra S. K., and Joore M. A.. 2005. “Joint Recovery Programme versus Usual Care: An Economic Evaluation of a Clinical Pathway for Joint Replacement Surgery.” Medical Care 43: 1018–26. [DOI] [PubMed] [Google Scholar]
- Carlsen, F. , Grytten J., Kjelvik J., and Skau I.. 2007. “Better Primary Physician Services Lead to Fewer Hospital Admissions.” European Journal of Health Economics: HEPAC: Health Economics in Prevention and Care 8: 17–24. [DOI] [PubMed] [Google Scholar]
- Chamberlain, G. 1992. “Comment: Sequential Moment Restrictions in Panel Data.” Journal of Business and Economic Statistics 10: 20–6. [Google Scholar]
- Contoyannis, P. , Jones A., and Rice N.. 2004. “The Dynamics of Health in the British Household Panel Survey.” Journal of Applied Econometrics 19: 473–503. [Google Scholar]
- De Lepeleire, J. , Iliffe S., Mann E., and Degryse J. M.. 2009. “Frailty: An Emerging Concept for General Practice.” British Journal of General Practice 59: e177–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusheiko, M. , Gravelle H., Martin S., Rice N., and Smith P. C.. 2011. “Does Better Disease Management in Primary Care Reduce Hospital Costs? Evidence from English Primary Care.” Journal of Health Economics 30: 919–32. [DOI] [PubMed] [Google Scholar]
- Fiorentini, G. , Iezzi E., Lippi Bruni M., and Ugolini C.. 2011. “Incentives in Primary Care and Their Impact on Potentially Avoidable Hospital Admissions.” European Journal of Health Economics: HEPAC: Health Economics in Prevention and Care 12: 297–309. [DOI] [PubMed] [Google Scholar]
- Fitzgerald, J. , Gottschalk P., and Moffitt R.. 1998. “The Impact of Attrition in the Panel Study of Income Dynamics on Intergenerational Analysis.” Journal of Human Resources 33: 300–44. [Google Scholar]
- Forder, J. 2009. “Long‐Term Care and Hospital Utilisation by Older People: An Analysis of Substitution Rates.” Health Economics 18: 1322–38. [DOI] [PubMed] [Google Scholar]
- Freedman, V. A. , Martin L. G., Schoeni R. F., and Cornman J. C.. 2008. “Declines in Late‐Life Disability: The Role of Early‐ and Mid‐Life Factors.” Social Science and Medicine 66 (7): 1588–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried, L. P. , Tangen C. M., Walston J., Newman A. B., Hirsch C., Gottdiener J., Seeman T., Tracy R., Kop W. J., Burke G., and McBurnie M. A.. 2001. “Frailty in Older Adults Evidence for a Phenotype.” The Journals of Gerontology Series A: Biological Sciences and Medical Sciences 56 (3): M146–57. [DOI] [PubMed] [Google Scholar]
- Fried, L. P. , Ferrucci L., Darer J., Williamson J. D., and Anderson G.. 2004. “Untangling the Concepts of Disability, Frailty, and Comorbidity: Implications for Improved Targeting and Care.” The Journals of Gerontology Series A: Biological Sciences and Medical Sciences 59 (3): M255–63. [DOI] [PubMed] [Google Scholar]
- Greenacre, M. , and Blasius J. (Eds.). 2006. Multiple Correspondence Analysis and Related Methods. Boca Raton, FL: CRC Press. [Google Scholar]
- Heckman, J. 1981. The Incidental Parameters Problem and the Problem of Initial Conditions in Estimating a Discrete Time‐Discrete Data Stochastic Processs. Cambridge, MA: MIT Press. [Google Scholar]
- Hughes, D. , and McGuire A.. 2003. “Stochastic Demand, Production Responses and Hospital Costs.” Journal of Health Economics 22: 999–1010. [DOI] [PubMed] [Google Scholar]
- Ilinca, S. , and Calciolari S.. 2015. “The Patterns of Health Care Utilization by Elderly Europeans: Frailty and Its Implications for Health Systems.” Health Services Research 50: 305–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn, J. L. , and Liu J. S.. 2013. “The Dynamics of Medical Care Use in the British Household Panel Survey.” Health Economics 22: 687–710. [DOI] [PubMed] [Google Scholar]
- Lang, P. O. , Michel J. P., and Zekry D.. 2009. “Frailty Syndrome: A Transitional State in a Dynamic Process.” Gerontology 55: 539–49. [DOI] [PubMed] [Google Scholar]
- Lee, Y. 2005. Specification Testing for Functional Forms in Dynamic Panel Data Models. Economics and Finance Workshop Discussion Paper Series. University of Hong Kong, School of Economics and Finance. [Google Scholar]
- McAdams‐DeMarco, M. A. , Law A., Salter M. L., Boyarsky B., Gimenez L., Jaar B. G., Walston J. D., and Segev D. L.. 2013. “Frailty as a Novel Predictor of Mortality and Hospitalization in Individuals of All Ages Undergoing Hemodialysis.” Journal of the American Geriatrics Society 61: 896–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijman, T. , and Verbeek M.. 1992. “Nonresponse in Panel Data: The Impact on Estimates of a Life Cycle Consumption Function.” Journal of Applied Econometrics 7 (3): 243–57. [Google Scholar]
- Prince, M. J. , Reischies F., Beekman A. T., Fuhrer R., Jonker C., Kivela S. L., Lawlor B. A., Lobo A., Magnusson H., Fichter M., van Oyen H., Roelands M., Skoog I., Turrina C., and Copeland J. R.. 1999. “Development of the EURO‐D Scale–A European, Union Initiative to Compare Symptoms of Depression in 14 European Centres.” British Journal of Psychiatry: The Journal of Mental Science 174: 330–8. [DOI] [PubMed] [Google Scholar]
- Santos‐Eggimann, B. , Cuenoud P., Spagnoli J., and Junod J.. 2009. “Prevalence of Frailty in Middle‐Aged and Older Community‐Dwelling Europeans Living in 10 Countries.” Journals of Gerontology 64: 675–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subra, J. , Gillette‐Guyonnet S., Cesari M., Oustric S., and Vellas B.. 2012. “The Integration of Frailty Into Clinical Practice: Preliminary Results from the Gerontopole.” Journal of Nutrition, Health & Aging 16: 714–20. [DOI] [PubMed] [Google Scholar]
- Wooldridge, J. 2005. “Simple Solutions to the Initial Conditions Problem in Dynamic, Nonlinear Panel Data Models with Unobserved Heterogeneity.” Journal of Applied Econometrics 20: 39–54. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix SA1: Author Matrix.
Table A1: Descriptive Statistics by Country and Waves (Average Values).


