Abstract
Background
ABO compatibility creates a disadvantage for O and B renal allograft candidates. A2 ABO incompatible transplant may decrease waiting times and generate equivalent graft survival to an ABO compatible transplant.
Methods
Death-censored graft survival was compared between A recipients and O, B, and AB recipients of an A2 allograft with multivariate Cox regression models utilizing data from the United Network of Organ Sharing (UNOS) between 1997 and 2007.
Results
Eighty-five percent of A2 kidneys were transplanted into ABO compatible recipients vs. 15% into ABO incompatible recipients. Rates of A2 incompatible kidney transplants did not increase over the study period (14.8% to 14.6%). Mean wait time for A2 → O kidneys was 337 vs. 684 d for O → O and for A2 → B kidneys, 542 vs. 734 d for B → B. Adjusted relative risk of graft loss at five-yr was similar between O, B, and AB recipients compared to A recipients of an A2 allograft, corresponding to a five-yr graft survival of 84%, 86.2%, 86.1%, and 86.1%, respectively.
Conclusion
A2 incompatible kidney transplantation is underutilized. Graft outcomes are similar among A2 compatible and incompatible recipients. Shorter waiting time and improved access might be achieved if A2 kidneys are considered in all blood groups.
Keywords: A2 blood group kidney, ABO incompatible transplantation, kidney transplantation, long-term graft outcomes, waiting time
The waiting list for deceased donor kidney transplantation has continued to grow. For kidney recipients listed in 2004, the median waiting time for patients with blood groups B or O was 1568 and 1655 d, compared to shorter median wait list times for A and AB, which were 815 and 602 d, respectively (1). The requirement for blood group compatibility is partly responsible for these differences in wait times, thus creating a disadvantage for O and B renal allograft candidates. This is particularly true among minorities, especially African Americans, who contribute a disproportionate number of patients on the blood type B waiting list (2, 3).
ABO blood subgroup A2 kidneys are found to have reduced expression of antigen on endothelial surfaces and are thus speculated to “act” more like an O allograft, allowing for a situation where ABO incompatible kidney transplant can occur with reproducible success (4, 5). Several reports have documented the successful transplantation of A2 kidney allografts across blood types with similar results to ABO compatible kidney transplants (6–8). Despite an A2 to B voluntary variance introduced by the United Network of Organ Sharing (UNOS) in 2001, few donor service areas have adopted it (9). Nelson et al. from the Midwest Organ Bank has reported a one- and five-yr graft survival among 41 B recipients of A2 and A2B kidneys of 91% and 85%. This was not significantly different from 80 B recipients of B or O kidneys who had a 91% and 80% one- and five-yr survival (10). These reports have demonstrated decreased waiting times among individual centers or donor service areas (11, 12).
Only 56 deceased donor A2 kidneys were reported from the UNOS database transplanted into other blood types between 1995 and 2003 (13). Because there is potential for decreasing wait time and increasing the transplant rate among the African American community, we sought to examine whether there had been an increase in the use of A2 kidneys for non-A recipients since this initial report. Furthermore, we sought to examine the potential to decrease waiting time for blood group B recipients, and in an adjusted analysis, examine graft survival among B and O recipients of an A2 allograft compared to A or AB recipients of an A2 allograft.
Methods
The study is a retrospective cohort analysis of kidney recipients between 1997 and 2007 using registry data from UNOS. The Hospital of the University of Pennsylvania Institutional Review Board approved the study.
The primary end point of the study was death-censored graft failure. Multiple organ transplant recipients were excluded. Time to allograft loss was calculated from transplantation until the date of allograft loss reported to UNOS. Multivariable Cox regression models were constructed to compare death-censored graft loss between blood type recipients A, O, AB, and B of A2 grafts. Independent variables nominally associated with the outcome (p < 0.10) in univariate analysis were entered into the multivariable models. Covariates not meeting entry threshold were also included if considered clinically significant. The model included the following covariates: recipient and donor age and race, recipient hepatitis C virus (HCV) status, diabetes, hypertension, number of previous kidney transplants, extended criteria donor (ECD) status, peak panel reactive antibody (PRA), transplant year, delayed graft function, degree of HLA match, cold ischemic time, and living or deceased donor.
Missing data included HCV status in 8.4% of recipients, diabetes status in 2.9%, cold ischemic time in 13.7%, PRA in 13.6%, and HLA match in 0.3%. In our primary analyses, multivariate models were constructed using patients with complete data. To demonstrate that eliminating patients with incomplete data did not significantly affect the primary relationships of interest, we conducted sensitivity analyses with imputation of missing covariates for variables missing >5% of data. Sensitivity analyses were performed in which missing values for PRA and cold ischemic time were imputed using values corresponding to the 90%, 10%, and mean among patients who did have data for these variables. Missing values for HCV were imputed as being either positive or negative. These secondary analyses demonstrated similar results as the primary analyses and are not shown. Means of variables were compared between groups using a one-way ANOVA. All analyses were performed using SPSS version 17.0 (SPSS Inc, Chicago, IL, USA).
Results
From January 1997 to November 2007, there were 1606 kidney transplants performed where the donor was identified as ABO subgroup A2. The annual proportion of kidney transplants in the United States with A2 organs that were compatible (A2 into A and A2 into AB) or incompatible (A2 into O and A2 into B) increased slightly from 0.9% to 1.2% of all kidney transplants between 1997 and 2007. Among all kidney transplants where the donor was identified as blood group A, the percentage identified as subtype A2 increased from 2.9% in 1997 to 3.6% in 2007.
Eighty-five percent of all A2 allografts over the study period were transplanted into ABO compatible recipients, and only 15% were transplanted into ABO incompatible recipients. The percentage of A2 kidneys used for ABO incompatible kidney transplants decreased slightly during the time frame of this analysis, 14.8% in the first year to 14.6% in the last year of this study.
Of the A2 donors, 14.6% were living donors and 85.4% deceased donors. Of the A2 compatible transplants, 88.5% were deceased donor and 11.5% were living donor transplants; among the A2 incompatible transplants, 52.3% were deceased donor and 47.3% were living donor transplants. We observed a significant increase in the percentage of living donors for A2 incompatible kidney transplant, increasing from 4.3% to 11.3% over the study period (p < 0.001).
All 11 UNOS regions used A2 kidneys for ABO incompatible transplants; however, the number of A2 incompatible kidney transplants varied greatly among UNOS regions (Table 1). The largest numbers of A2 kidneys were identified in region 2, with only 6.7% of A2 kidneys in this region going to ABO incompatible recipients. This compared to region 8, which also identified a large number of A2 donors, but 46.2% went to ABO incompatible recipients (Table 1).
Table 1.
A2 kidney transplants from 1997 to 2007 by UNOS region
| A2 kidney transplants by region | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Region | A2 per region | Total number of A (%A2) | A2 to A per region | A2 to AB per region | A2 to O per region | A2 to B per region |
| 1 | 20 | 2307 (0.9) | 9 | 0 | 10 | 1 |
| 2 | 315 | 7404 (4.3) | 267 | 27 | 9 | 12 |
| 3 | 201 | 6367 (3.2) | 177 | 14 | 2 | 8 |
| 4 | 117 | 3800 (3.1) | 95 | 6 | 6 | 10 |
| 5 | 218 | 7143 (3.1) | 178 | 7 | 27 | 6 |
| 6 | 109 | 2086 (3.2) | 72 | 7 | 9 | 21 |
| 7 | 114 | 5581 (2.0) | 87 | 6 | 17 | 4 |
| 8 | 160 | 3305 (4.8) | 83 | 3 | 22 | 52 |
| 9 | 120 | 3275 (3.7) | 103 | 3 | 13 | 1 |
| 10 | 133 | 5082 (2.6) | 127 | 3 | 3 | 0 |
| 11 | 99 | 4756 (2.1) | 88 | 3 | 7 | 1 |
| Total | 1606 | 51109 | 1286 | 79 | 125 | 116 |
UNOS, United Network of Organ Sharing.
The distribution of recipient and donor characteristics of specific interest appears in Tables 2 and 3. The mean waiting time for an incompatible transplant using A2 kidneys was significantly less than for ABO compatible kidneys: the mean waiting time for blood type O recipients of an A2 kidney was 337 d as compared to 684 d for O recipients of ABO compatible kidneys (p < 0.001), and the mean wait time for B recipients of A2 kidneys was 542 d as compared to 734 d for B recipients of ABO compatible kidneys (p < 0.001). Examining wait times for just deceased donor organs reveals a greater difference: the mean waiting time for blood type O recipients of an A2 deceased donor kidney was 360 d as compared to 832 d for O recipients of ABO compatible deceased donor kidneys (p < 0.001). The mean wait time for B recipients of A2 deceased donor kidneys was 540 d as compared to 907 d for B recipients of ABO compatible deceased donor kidneys (p < 0.001) (Table 2).
Table 2.
A2 recipient characteristics from 1997 to 2007
| Recipient characteristics | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| A2 to A | A2 to AB | A2 to O | A2 to B | Total | p-value | |
| Mean age of recipient – yr (SD) | 49 (15) | 47 (16.1) | 42 (15.8) | 50 (13.2) | 49 (15.1) | <0.001 |
| Gender of recipient | ||||||
| Male (%) | 775 (58.7) | 40 (50.6) | 65 (52.0) | 74 (63.8) | 934 (58.2) | 0.141 |
| Race of recipient | ||||||
| White (%) | 832 (64.7) | 43 (54.4) | 99 (79.2) | 55 (47.4) | 1029 (64.1) | <0.001 |
| Black (%) | 269 (20.9) | 24 (30.4) | 12 (9.6) | 35 (30.2) | 340 (21.2) | |
| Other (%) | 185 (14.4) | 12 (15.2) | 14 (11.2) | 26 (22.4) | 237 (14.8) | |
| HLA match (%) | ||||||
| 0 | 187 (14.5) | 8 (10.1) | 11 (8.8) | 28 (24.1) | 234 (14.6) | <0.001 |
| 1 | 324 (25.2) | 24 (30.4) | 26 (20.8) | 41 (35.3) | 415 (25.8) | |
| 2 | 270 (21.0) | 16 (20.3) | 16 (12.8) | 32 (27.6) | 334 (20.8) | |
| 3 | 252 (19.6) | 13 (16.5) | 48 (38.4) | 12 (10.3) | 325 (20.2) | |
| 4 | 109 (8.5) | 7 (8.9) | 15 (12.0) | 3 (2.6) | 134 (8.3) | |
| 5 | 86 (6.7) | 6 (7.6) | 4 (3.2) | 0 | 96 (6.0) | |
| 6 | 55 (4.3) | 5 (6.3) | 3 (2.4) | 0 | 63 (3.9) | |
| HCV (%) | 68 (5.3) | 8 (10.1) | 2 (1.6) | 6 (5.2) | 84 (5.2) | 0.264 |
| Number of previous kidneys (%) | ||||||
| 0 | 1159 (90.1) | 62 (78.5) | 113 (90.4) | 103 (88.8) | 1437 (89.5) | 0.021 |
| 1 | 117 (9.1) | 16 (20.3) | 11 (8.8) | 13 (11.2) | 157 (9.8) | |
| ≥2 | 10 (0.8) | 1 (1.3) | 1 (0.8) | 0 | 12 (0.7) | |
| Delayed graft function (%) | 337 (26.2) | 15 (19) | 8 (6.4) | 25 (21.6) | 385 (24) | <0.001 |
| Diabetes (%) | 381 (29.6) | 34 (43) | 34 (27.2) | 41 (35.3) | 490 (30.5) | 0.046 |
| Peak PRA – mean (SD) | 16.0 (29.3) | 22.8 (35.4) | 13.81 (26.8) | 9.31 (21.1) | 15.7 (29.1) | 0.018 |
| Average wait time – d | ||||||
| All A2 (SD) | 575 (602) | 399 (444) | 337 (328) | 542 (548) | 552 (586) | <0.001 |
| Deceased donor A2 (SD) | 587 (417) | 405 (234) | 360 (280) | 540 (568) | 572 (392) | <0.001 |
| All ABO compatiblea (SD) | 494 (518) | 382 (432) | 684 (657) | 734 (521) | 606 (615) | <0.001 |
| Deceased donor ABO compatible (SD) | 583 (555) | 417 (456) | 832 (693) | 907 (721) | 726 (654) | <0.001 |
| Induction immunosuppressionb,c % | 54 | 57 | 55 | 67 | 55 | 0.147 |
| Discharge immunosuppressionb | ||||||
| Tacrolimus % | 45 | 43 | 37 | 57 | 45 | <0.001 |
| Cyclosporine % | 44 | 38 | 49 | 38 | 45 | |
| Mycophenolate % | 60 | 58 | 57 | 80 | 57 | |
| Imuran % | 28 | 35 | 31 | 19 | 34 | |
| Rapamycin % | 5 | 2 | 8 | 6 | 5 | |
| Rejection within 1 yr (%) | 179 (13.9) | 8 (10.1) | 11 (8.8) | 14 (11.2) | 212 (13) | 0.325 |
HCV, hepatitis C virus; PRA, panel reactive antibody.
Wait time for recipients of ABO compatible kidneys for blood groups A, AB, O and B, respectively.
Percentage of A2 recipients that immunosuppression data were reported, missing data for 40.1% of recipients.
Induction with either OKT3, Thymoglobulin, Simulect or Campath.
Table 3.
Donor characteristics from 1997 to 2007
| Donor characteristics | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| A2 to A | A2 to AB | A2 to O | A2 to B | Total | p-value | |
| Mean age of donor – yr (SD) | 40 (16) | 37.7 (16.7) | 42 (12) | 33 (15.5) | 40 (16.0) | <0.001 |
| Gender of donor | ||||||
| Male (%) | 712 (55.4) | 48 (60.8) | 56 (44.8) | 53 (45.7) | 869 (54.1) | 0.019 |
| Race of donor | ||||||
| White (%) | 1112 (86.5) | 70 (88.6) | 102 (81.6) | 107 (92.2) | 1391 (86.6) | 0.133 |
| Black (%) | 64 (5.0) | 5 (6.3) | 8 (6.4) | 2 (1.7) | 79 (4.9) | |
| Other (%) | 110 (8.6) | 4 (5.1) | 15 (12.0) | 7 (6.0) | 136 (8.5) | |
| Hypertension (%) | 331 (25.7) | 17 (21.5) | 1 (0.8) | 13 (11.2) | 362 (22.5) | <0.001 |
| Cold ischemic time – h (SD) | 17.9 (9.3) | 15.6 (8.6) | 3.6 (6.1) | 13.2 (6.6) | 16.6 (9.6) | <0.001 |
| Type of donor | ||||||
| Living (%) | 103 (8.0) | 6 (7.6) | 112 (89.6) | 14 (12.1) | 235 (14.6) | <0.001 |
| Deceased (%) | 1183 (92.0) | 73 (92.4) | 13 (10.4) | 102 (87.9) | 1371 (85.4) | |
| Donor ECD (%) | 588 (45.7) | 34 (43) | 7 (5.6) | 42 (36.2) | 671 (41.8) | <0.001 |
| Diabetes (%) | 64 (5) | 6 (7.6) | 0 | 1 (0.9) | 76 (4.4) | 0.046 |
ECD, extended criteria donor.
Given that A2 incompatible transplant is being encouraged to relieve the wait time burden among blood group B African American transplant candidates, we examined the proportion of African American recipients of A2 incompatible transplants compared to those receiving an ABO compatible organ. Thirty percent (88.6% deceased donors and 11.4% living donors) of blood group B recipients of A2 kidneys were African American compared to 34% (71.3% deceased donors and 28.7% living donors) of blood group B recipients of an ABO compatible kidney. The mean wait time for African American recipients of an A2 incompatible kidney was significantly shorter than those of an ABO compatible kidney [645 vs. 792 d, (p < 0.001)]. When this analysis was repeated by excluding living donors, African American recipients of a deceased donor incompatible kidney experienced an even greater relative reduction in wait time when compared to recipients of a compatible graft, 663 vs. 882 d (p < 0.001).
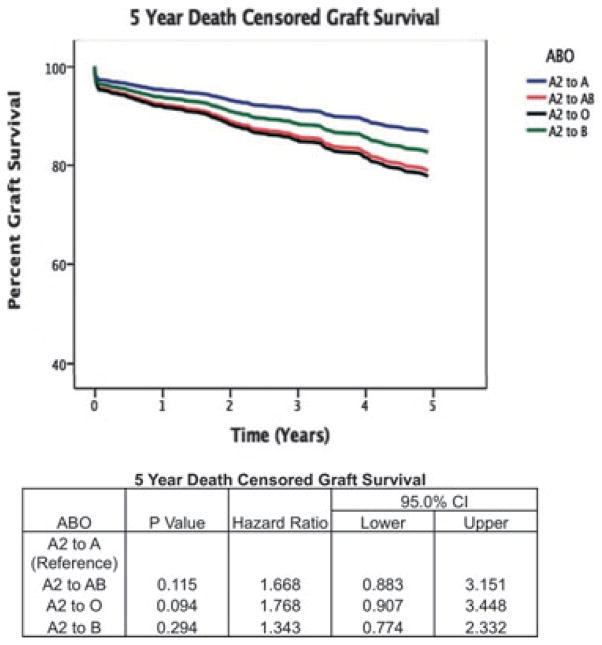
Using Cox regression, we determined that the adjusted relative risk of death-censored graft loss at one-yr was not statistically different among O recipients (p = 0.073, HR = 3.62, CI = 0.88–14.8), B recipients (p = 0.40, HR = 1.38, CI = 0.65–3.44), and AB recipients (p = 0.59, HR = 1.29, CI = 0.50–3.35), compared to A recipients of an A2 kidney, and this corresponded to a one-yr graft survival of 92.0, 92.2, 93.7, and 93.0%, respectively. Similarly, we determined that the adjusted relative risk of death-censored graft loss at five-yr was not statistically different among O (p = 0.09 and HR = 1.77, CI = 0.91–3.45), B (p = 0.29 and HR = 1.34, CI = 0.77–2.33), and AB (p = 0.12 and HR = 1.67, CI = 0.88–3.15), compared to A recipients of an A2 kidney, and this corresponded to a five-yr graft survival of 84.0%, 86.2%, 86.1%, and 86.1%, respectively (Fig. 1).
Fig. 1.
Five year death censored graft survival estimated by Cox regression analysis.
We repeated this regression analysis to include only deceased donor kidneys, given that A2 incompatible transplant is being encouraged to relieve the wait time burden. Neither the one- or five-yr adjusted relative risk of death-censored graft loss was different among O recipients (p = 0.92, HR = 1.10, CI = 0.15–7.90 one-yr; p = 0.47, HR = 1.67, CI = 0.40–7.10 five-yr), B recipients (p = 0.28, HR = 1.40, CI = 0.73–3.03 one-yr; p = 0.13, HR = 1.54, CI = 0.89–2.90 five-yr), and AB recipients (p = 0.96, HR = 1.02, CI = 0.42–2.53 one-yr; p = 0.27, HR = 1.50, CI = 0.72–3.14 five-yr) compared to A recipients of an A2 kidney. This corresponded to a one- and five-yr graft survival of 92.3% and 84.6% for O recipients, 93.1% and 87.3% for B recipients, 93.2% and 86.3% for AB recipients, and 92.6% and 85.7% for A recipients, respectively.
Discussion
In this retrospective study examining the outcomes of A2 ABO compatible and incompatible kidney transplantation, we demonstrate in an adjusted analysis that the long-term outcomes of A2 incompatible kidney transplantation appear to be similar to A2 ABO compatible kidney transplantation. This analysis confirms prior reports that demonstrate equivalent outcomes of A2 kidneys into ABO incompatible recipients (6, 8, 14–17). Nelson et al. (15) reported one of the largest series: a 10-yr multicenter experience with transplantation of 50 blood group A2 kidneys into B and O patients. Two-yr deceased donor graft survival was 94%. Our analysis incorporates these A2 transplants and identifies a total of 1606 A2 kidneys that were subtyped over a 10-yr period. Although not statistically significant, we did find a trend toward inferior one-yr graft survival for A2 to O recipients. This could be related to the higher anti-A titers known to be found in O recipients when compared to B recipients (16).
The present analysis confirms decreased waiting time for both B and O recipients of A2 kidneys. Minorities, both African Americans and Asians, stand to benefit from these reductions in wait time given that minority candidates comprise two-thirds of the national blood group B waiting list. Despite the potential for assisting minority populations, African Americans appear to be transplanted less frequently with A2 incompatible transplants than one would expect: 30% of blood group B recipients of A2 kidneys were African American compared to 34% of blood group B recipients of an ABO compatible kidney. Given that the UNOS variance was created to increase access to kidney transplantation for blood group B African Americans, one would expect an even larger percentage of blood group B African Americans that receive A2 kidneys, not less. One possible explanation is that African Americans could have higher anti-A titers than Caucasians and are excluded from transplant; however, an analysis by Bryan et al. (11) demonstrated no difference in anti-A titers between black and white recipients. Additionally, it could be that centers that embrace this approach simply have lower number of African Americans listed, which is the case for region (8). While the reasons for the underutilization of A2 kidneys in African Americans recipients are unclear, incorporating emphasis as to the benefits of A2 incompatible kidney transplantation into transplant candidate education may increase the number of African American who consider this option.
While 20% of A type donors bear A2 in large typing studies (11), far lower percentages were identified as A2 to UNOS during the study period (3.1%), indicating that subtyping is not being performed routinely and that most patients are simply assigned blood group A. Despite recommendations from UNOS that all ABO blood group A donors be subtyped, we only observed a marginal increase in A2 subtyping during the study period, with only 3.6% of blood group A organs identified as A2 in the final year of the study. Furthermore, the use of A2 typing, despite occurring in every UNOS region, varies considerably, ranging from 0.9% to 5.2% of all blood group A donors in the eleven UNOS regions. Additionally, the use of A2 kidneys in ABO incompatible transplant settings varies widely with UNOS region: in region 2, 6.7% of A2 kidneys are utilized in ABO incompatible transplants compared to region 8 were 46.2% of A2 kidneys are used in ABO incompatible transplants. Nationally, A2 ABO incompatible transplantation has not increased during the study period, in fact it has decreased slightly from 14.8% in the first year to 14.6% in the last year of this study. The lack of increase in A2 incompatible transplantation exists in spite of the A2 to B UNOS approved kidney allocation variance. Even with the variance and recommendations from UNOS to subtype A donors, our analysis shows that the majority of A2 kidneys, when they are identified, are being transplanted into A recipients.
Significant barriers exist to further expand the use of A2 donors to decrease waiting times in ABO blood group B minority populations; we offer the following recommendations. Blood group A2 subtyping is not being conducted routinely as recommended by UNOS. Not all hospitals have this capability, and organ procurement organizations should partner with the donor hospitals to increase typing among all deceased donors. Few organ procurement organizations have utilized the A2 to B variance approved by UNOS. Transplant programs should work with their organ procurement organizations to establish mechanisms to allow blood group O or B candidates with long waiting times to allow access to an A2 kidney. From a recipient standpoint, blood group B kidney transplant candidates should be educated about the potential benefit of receiving an A2 kidney and transplant programs need to be able to perform anti-A titers. Finally, one might argue that A2 incompatible transplants could best be utilized in the living donor setting, where they can be a solution for the donor organ that goes un-transplanted for incompatibility, particularly if participation occurs within a kidney donor exchange or chain.
Certain limitations in our analysis exist. As with other analyses performed with the UNOS data set, we have encountered missing data. As such we have addressed this issue using imputation to demonstrate that the results of the multivariable analysis are stable. In all analyses, there may be unmeasured covariates that are important to the outcome of ABO incompatible transplantation. Certainly, there may be a selection bias in why outcomes are similar between A2 compatible and incompatible transplants. It has been demonstrated that outcomes of A2 to O incompatible kidney transplant improve when anti-A IgG titers are 1:8 or less (7, 16). Unfortunately, the UNOS database does not record anti-A titers and has very limited data with regard to immunosuppression regimens, which are important to consider when evaluating the outcome of ABO incompatible transplantation.
From our analysis, we conclude that A2 ABO incompatible kidney transplantation is significantly underutilized, despite similar long-term graft survival to ABO compatible kidney transplantation. Our analysis provides further evidence that improved access to transplantation and shorter waiting time will be achieved if A2 kidneys are considered in all blood groups, especially for African Americans.
Acknowledgments
This work was presented at the 2010 American Transplant Congress.
Footnotes
Conflict of interest: The authors report no conflict of interest.
References
- 1.2009 OPTN/SRTR Annual Report 1999–2008. HHS/HRSA/HSB/DOT.
- 2.Glander P, Budde K, Schmidt D, et al. The ‘blood group O problem’ in kidney transplantation – time to change? Nephrol Dial Transplant. 2010;25:198. doi: 10.1093/ndt/gfp779. [DOI] [PubMed] [Google Scholar]
- 3.Kasiske BL, Neylan JF, III, Riggio RR, et al. The effect of race on access and outcome in transplantation. N Engl J Med. 1991;324:302. doi: 10.1056/NEJM199101313240505. [DOI] [PubMed] [Google Scholar]
- 4.Breimer ME, Molne J, Norden G, Rydberg L, Thiel G, Svalander CT. Blood group A and B antigen expression in human kidneys correlated to A1/A2/B, Lewis, and secretor status. Transplantation. 2006;82:479. doi: 10.1097/01.tp.0000231697.15817.51. [DOI] [PubMed] [Google Scholar]
- 5.Breimer ME, Samuelsson BE. The specific distribution of glycolipid-based blood group A antigens in human kidney related to A1/A2, Lewis, and secretor status of single individuals. A possible molecular explanation for the successful transplantation of A2 kidneys into O recipients. Transplantation. 1986;42:88. [PubMed] [Google Scholar]
- 6.Alkhunaizi AM, de Mattos AM, Barry JM, Bennett WM, Norman DJ. Renal transplantation across the ABO barrier using A2 kidneys. Transplantation. 1999;67:1319. doi: 10.1097/00007890-199905270-00005. [DOI] [PubMed] [Google Scholar]
- 7.Nelson PW, Helling TS, Pierce GE, et al. Successful transplantation of blood group A2 kidneys into non-A recipients. Transplantation. 1988;45:316. doi: 10.1097/00007890-198802000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Bryan CF, Nelson PW, Shield CF, et al. Long-term survival of kidneys transplanted from live A2 donors to O and B recipients. Am J Transplant. 2007;7:1181. doi: 10.1111/j.1600-6143.2007.01750.x. [DOI] [PubMed] [Google Scholar]
- 9.Williams WW, Cherickh WS, Young C, et al. Update on the OPTN/UNOS national voluntary variance to allocate A2/A2B deceased donor kidneys to blood group B candidates. Am J Transplant. 2008;6:30. [Google Scholar]
- 10.Nelson PW, Shield CF, III, Muruve NA, et al. Increased access to transplantation for blood group B cadaveric waiting list candidates by using A2 kidneys: time for a new national system? Am J Transplant. 2002;2:94. doi: 10.1034/j.1600-6143.2002.020115.x. [DOI] [PubMed] [Google Scholar]
- 11.Bryan CF, Nelson PW, Shield CF, III, et al. Transplantation of A2 and A2B kidneys from deceased donors into B waiting list candidates increases their transplantation rate. Clin Transpl. 2004:127. [PubMed] [Google Scholar]
- 12.Bryan CF, Shield CF, III, Nelson PW, et al. Transplantation rate of the blood group B waiting list is increased by using A2 and A2B kidneys. Transplantation. 1998;66:1714. doi: 10.1097/00007890-199812270-00025. [DOI] [PubMed] [Google Scholar]
- 13.Futagawa Y, Terasaki PI. ABO incompatible kidney transplantation – an analysis of UNOS Registry data. Clin Transplant. 2006;20:122. doi: 10.1111/j.1399-0012.2005.00452.x. [DOI] [PubMed] [Google Scholar]
- 14.Bryan CF, Nelson PW, Aeder MI, et al. Current experience with renal transplantation across the ABO blood group barrier. Transplant Proc. 1992;24:2527. [PubMed] [Google Scholar]
- 15.Nelson PW, Landreneau MD, Luger AM, et al. Ten-year experience in transplantation of A2 kidneys into B and O recipients. Transplantation. 1998;65:256. doi: 10.1097/00007890-199801270-00020. [DOI] [PubMed] [Google Scholar]
- 16.Nelson PW, Helling TS, Shield CF, Beck M, Bryan CF. Current experience with renal transplantation across the ABO barrier. Am J Surg. 1992;164:541. doi: 10.1016/s0002-9610(05)81197-3. [DOI] [PubMed] [Google Scholar]
- 17.Sorensen JB, Grant WJ, Belnap LP, Stinson J, Fuller TC. Transplantation of ABO group A2 kidneys from living donors into group O and B recipients. Am J Transplant. 2001;1:296. doi: 10.1034/j.1600-6143.2001.001003296.x. [DOI] [PubMed] [Google Scholar]