Abstract
Tinea pedis (athlete's foot) and onychomycosis (infection of the toenails) caused by the dermatophyte fungus Trichophyton are highly prevalent in adults. Several Trichophyton allergens have been identified based on elicitation of immunoglobulin E antibody-mediated immediate-hypersensitivity (IH) responses. Evidence of an etiologic role for Trichophyton in asthma in some subjects with IH and chronic dermatophytosis is provided by bronchial reactivity to Trichophyton. Improvement of asthma after systemic antifungal treatment corroborates this link. A unique feature of Trichophyton allergens is the ability of the same antigen to elicit delayed-type hypersensitivity (DTH) in individuals who lack IH reactivity. Delayed responses appear to confer protection, while IH responses do not, based on the association with acute versus chronic skin infection. The amino acid sequence identity of Trichophyton allergens with diverse enzyme families supports a dual role for these proteins in fungal pathogenesis and allergic disease. Characterizing the immunologic properties of Trichophyton allergens and defining immune mechanisms which drive dichotomous responses are pivotal to understanding the dermatophyte-allergy relationship. Recent studies have identified DTH-associated major T-cell epitopes which could facilitate the development of peptide vaccines. Characterization of additional molecular targets by using new techniques may aid not only in the eradication of infection but also in the resolution of allergic symptoms.
INTRODUCTION
Skin and nail infections caused by dermatophyte fungi such as Trichophyton have become more common in recent years. Indeed, in some areas of the world, Trichophyton infection is now considered a major public health problem (36, 39, 46, 50). Dermatophytosis occurring in later life manifests most frequently as Trichophyton rubrum infection of the toenails and plantar surfaces of the feet (athlete's foot). Description of an association between allergic disease and chronic dermatophytosis dates back more than 70 years (120); however, only within the last two decades has a definitive link between sensitization to Trichophyton and asthma been established (115, 116). In this article, evidence is presented to support an etiologic role for Trichophyton infection in the development of asthma and other allergic diseases. The development of immediate hypersensitivity (IH) versus delayed-type hypersensitivity (DTH) to Trichophyton allergens may be pivotal to the course and severity of skin infection and also to the development of allergic disease. Definition of the molecular characteristics and immunologic properties of newly identified Trichophyton allergens has advanced our understanding of distinct immune responses to this pathogen (25, 108, 122-125). The putative function of Trichophyton allergens in fungal pathogenesis is described based on their sequence identity to known enzymes. Features of the T-cell repertoire associated with dichotomous immune responses to the same allergen are described, and the relevance of altered T-cell reactivity to IH and allergic disease is discussed. The potential for vaccine development using allergen-derived peptides containing DTH-associated epitopes is also presented. Application of new techniques holds great promise for identifying additional allergens and elucidating their biologic and immunologic characteristics. Such studies are fundamental to defining the determinants of protective immunity and to the design of treatments which could confer long-lasting resistance to infection.
DERMATOPHYTOSIS: AN INCREASING PROBLEM
Dermatophyte fungi of the genera Trichophyton, Epidermophyton, and Microsporum infect human skin, hair, and nails. These types of infections, termed “dermatophytoses,” are widespread and increasing in prevalence on a global scale. Infections of the feet are particularly troublesome and affect both the skin (athlete's foot) and nails (tinea unguium). Furthermore, despite the availability of new systemic antifungal therapies, nail infections are difficult to eradicate, with recurrence reported in up to 25 to 40% of cases (49). Dermatophytes account for the majority (90%) of cases of fungal nail infections (onychomycosis) in the United States and Europe (31). The overall prevalence of fungal nail infection reported as part of the U.S. Health and Nutrition Examination Survey (NHANES I) during the 1970s was relatively low (2.18%) (5a). By contrast, a more recent cross-sectional survey confirmed the presence of dermatophyte onychomycosis in 8.7% of patients attending a dermatology clinic in Cleveland, Ohio (28). Furthermore, a recent multicenter epidemiologic survey carried out in North America reported onychomycosis in 13.8% of participants (38). In that study, participants were patients attending primary-care physician offices or persons accompanying them during their visits. Thus, the relatively high prevalence of onychomycosis in these two studies may reflect selection of the study population. Previous studies carried out in Europe reported an incidence of onychomycosis ranging from 2.7 to 8.4% (43, 53, 96, 98). However, a recent report suggests that the prevalence of fungal foot disease may be much higher. The Achilles Project is a population-based two-part survey conducted in 16 European countries, in which all patients presenting to general practitioners and dermatologists were invited to participate (15). Results reported in 2003 showed that 34.9% of the 70,497 patients enrolled in study I had foot disease of fungal origin, with onychomycosis and tinea pedis (athlete's foot) being the most common. Thus, the incidence of fungal foot disease was strikingly high. There is no doubt that the different enrollment and diagnostic criteria for these studies could contribute to the variability in reported prevalence of onychomycosis. For example, questionnaire-based studies which are dependent on self-diagnosis have reported a relatively low prevalence of onychomycosis (<3%) (96, 98). By contrast, studies which utilized clinical diagnosis and microscopic examination or fungal culture yielded a higher prevalence of disease (6.86 to 34.9%) (15, 38, 45, 53). Regardless, epidemiologic studies point to an increase in the incidence of dermatophytosis since the 1970s.
Reasons for this increase are not clear, although it may be due, in part, to an aging population in North America. Indeed, an increase in infection with increasing age is well established (28, 43, 53, 96, 98, 107). This observation is consistent with the view that changes in the immune response which occur with advancing age lead to disease susceptibility. In keeping with this, fungal nail infections are more frequent in immunocompromised patients such as those who are human immunodeficiency virus (HIV) positive and those who have diabetes (34, 38, 44, 80). Alternatively, age-related changes in peripheral vasculature may be important in predisposing to infection (34). In addition to the effects of aging, genetic susceptibility has been proposed to contribute to infection. Although T. rubrum infection has been reported to show a familial pattern of autosomal dominant inheritance, more recent epidemiologic findings challenge this view (105, 128). Other factors which have been implicated include those associated with “modern life-style” including the use of footwear made from synthetic materials and exposure to dermatophytes in communal areas with damp environments which favor fungal growth, such as swimming pools and school gymnasiums. Despite the identification of multiple predisposing factors, there is no consensus of opinion regarding a single mechanism to explain the increased incidence of foot disease which has occurred in recent years.
Trichophyton species play a major role in dermatophyte infections of both the feet and skin. In North America, T. rubrum and T. mentagrophytes are the most frequent pathogens associated with onychomycosis (111). In countries with warm climates and other geographic regions, different dermatophyte species may be involved; however, T. rubrum remains one of the most frequent clinical isolates. For example, in Libya, yeasts of the genus Candida are the dominant cause of onychomycosis in women, with T. violaceum, T. rubrum, T. mentagrophytes and Microsporum canis being responsible for the majority of infections in men (30). Similar observations have also been reported from Pakistan (13). An increased incidence of dermatophyte infection in men compared with women is well documented. For example, in Spain the prevalence of tinea unguium and tinea pedis in men was ≥4%, compared with 1.7% for women (89). In that study, T. rubrum was the most common etiologic agent associated with both conditions. Increased dermatophyte infection in men has also been reported in a multicenter survey carried out in North America, as well as among atopic and nonatopic subjects in Venezuela and among diabetic subjects in Canada (33, 38, 44). Thus, a propensity for men to develop dermatophytosis appears to be a widespread phenomenon.
Although foot infections are more common in aging adults, pathogenic dermatophytes can be present on children's feet. For example, pathogenic dermatophytes were isolated from the feet of 21% of Mexican children ages 2 to 12 years who presented with plantar and/or interdigital scaling, maceration, and pruritus (9). In the same study, dermatophytes were also cultured from 7% of children with apparently healthy feet, suggesting a high carrier rate. Nevertheless, the prevalence of onychomycosis or tinea pedis in children compared with adults is markedly reduced in Canada and the United States (0.16%) (45). Instead, dermatophytosis in children usually manifests as infections of the scalp (tinea capitis) caused by M. canis and T. tonsurans. Similar to the increased prevalence of foot disease reported in adults, there has been a large increase in infections caused by T. tonsurans, with this species surpassing M. canis as the primary etiologic agent in urban settings. Indeed, scalp ringworm is reaching epidemic proportions in urban areas of Europe and both North and South America, with a predominance in children of African Caribbean or African American descent (14, 36, 39, 46, 50). Thus, exposure to Trichophyton during early life is widespread, and this may have a profound effect on the response to subsequent infection occurring in later life.
CLINICAL PRESENTATION AND TRANSMISSION
Dermatophyte organisms associated with onychomycosis invade the nail plate as well as the surface of the nail itself, most frequently resulting in yellow/brown discoloration, ridging, and thickening (hyperkeratosis). Onychomycosis has been classified into four distinct forms: distal subungual, proximal subungual, white superficial, and Candida nail infections (27). Distal subungual onychomycosis is most common, with T. rubrum being the primary cause. This infection is characterized by invasion of the most distal portion of the nail bed, which then migrates proximally. Eventually the nail may separate from the nail bed (onycholysis), allowing secondary infection by bacteria and other molds. Proximal subungual onychomycosis is uncommon in the general population but common in AIDS patients. In this infection, the organism invades the newly formed nail plate, causing whitish discoloration of the nail (leukonychia), which migrates distally. White superficial onychomycosis is less common than distal subungual onychomycosis and results from infection in the superficial layers of the nail plate rather than in the nail bed. Candida nail infections may be confused with other onychomycoses since they can present in a similar manner; however, infection starts in structures surrounding the nail before invasion of the nail plate.
Trichophyton infections of the skin of the feet (tinea pedis or athlete's foot) frequently accompany onychomycosis. These conditions, along with infections of the groin (tinea cruris), are relatively common among men. In addition, infection of the upper body (tinea corporis gladiatorum) is an emerging problem among adolescents, with a prevalence of 24 to 77% among individuals in wrestling teams (2, 11, 52). This condition, which is transmitted by skin-to-skin contact, is most commonly associated with T. tonsurans in the United States (72). Interestingly, infections of the scalp (tinea capitis) are also caused primarily by T. tonsurans, and it is thought that asymptomatic scalp infections may be a reservoir for transmission. The features of skin lesions vary depending on the causative agent and the site affected. For example, infections of the feet tend to be chronic and can be scaling, vesicular, or ulcerative in form. Such infections may result in hyperkeratosis of the plantar surface of the feet. Scaling can occur with or without erythema and may be restricted to the lateral aspect of the sole, the instep, or the interdigital area. Tinea corporis typically presents as well-defined erythematous, scaling plaques on the arms, legs, and trunk, and lesions often assume a circular “ringworm” shape. However, the distribution of tinea corporis gladiatorum is different, affecting the head, neck, and upper extremities but rarely the legs (3). Scalp infections may result in kerion formation, characterized by a raised, tender mass of inflamed tissue. The type of infecting agent may also contribute to the clinical picture. For example, zoophilic species of dermatophytes, which are found predominantly in animals (e.g., T. mentagrophytes var. mentagrophytes and var. erinacei, T. verrucosum, and M. canis), often cause highly inflamed lesions in humans. In contrast, the anthropophilic species which typically colonize humans (e.g., T. rubrum, T. mentagrophytes var. interdigitale, and T. tonsurans) tend to be associated with more chronic infections which are less inflammatory in nature. A feature that all dermatophyte fungi have in common is their ability to utilize keratin as a nutrient source. These fungi therefore differ from most other pathogenic fungi since they are not opportunists and frequently infect healthy individuals. Considering the high prevalence of dermatophytosis in the general population, dermatophyte fungi appear to occupy a niche which is optimal to their survival and growth in the host-pathogen relationship.
IMMUNE RESPONSE TO DERMATOPHYTE FUNGI
Immediate and Delayed-Type Hypersensitivity Reactions to Trichophyton
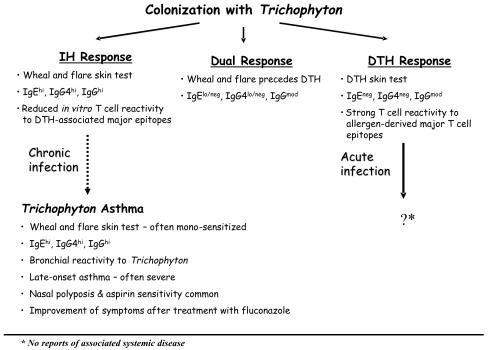
Antigens derived from Trichophyton exhibit unusual immunologic properties based on their ability to induce distinct skin test reactions in different individuals (Fig. 1). IH skin tests to Trichophyton extract are frequently observed in subjects with chronic dermatophytosis. These immunoglobulin E (IgE) antibody (Ab)-mediated reactions are characterized by a local wheal and flare occurring 5 to 20 min after injection of antigen into the skin. In this process, binding of antigen to IgE Ab on the surface of mast cells results in cross-linking of IgE Ab, which, in turn, triggers the degranulation of mast cells and release of histamine and other proinflammatory mediators. IH skin tests to Trichophyton are associated with the presence of serum IgE Ab and IgG Ab to Trichophyton antigens (25, 123). Furthermore, IgG4 Ab are a major component of the IgG Ab response (123). Thus, IH reactions to dermatophytes bear the hallmarks of a Th2 response. In this type of response, interleukin-4 (IL-4) produced by CD4+ T cells (Th2 cells) induces antibody isotype switching to IgG4 and IgE (Fig. 1).
FIG. 1.
Trichophyton infection results in the development of distinct immune responses. IH responses are characterized by a wheal-and-flare skin test and the presence of high-titer IgE and IgG4 Ab to Trichophyton extract and purified allergens in serum. IH responses are frequently observed in subjects presenting with chronic infection. A minority of subjects with chronic dermatophytosis who are sensitized to Trichophyton exhibit features of Trichophyton asthma. A dual response is characterized by a wheal and flare which precedes a DTH response. IgE Ab may be a component of this response. Delayed reactions occurring in the absence of IH are typically maximal at 24 to 48 h after intradermal injection of Trichophyton antigens. IgE antibodies are not a feature of DTH to Trichophyton, and there are no reports of associated systemic disease. DTH is associated with specific recognition of major T-cell epitopes derived from the T. rubrum allergen, Tri r 2 (124). A role for diminished T-cell responsiveness to DTH-associated major epitopes has been proposed to contribute to IH and chronic infection.
By contrast, DTH is a form of cell-mediated immunity in which the ultimate effector cell is the activated macrophage. In the classical DTH reaction, activation of macrophages is mediated by gamma interferon-(IFN-γ)-producing (Th1) CD4+ T lymphocytes. These T cells recognize and respond to foreign antigen presented in the form of peptide (T-cell epitopes) complexed with class II major histocompatibility complex molecules expressed on the surface of antigen-presenting cells (APCs). This cell-mediated response is characterized by induration at the injection site, which is maximal at 48 h. DTH to Trichophyton extract is associated with lower titers of IgG Ab to Trichophyton antigens and no IgE or IgG4 (Fig. 1) (25, 123). A subset of patients also mount “dual” skin test responses in which a DTH response follows the IH reaction. Whether this represents a transitional stage in DTH-to-IH conversion (or vice versa) is not known. Given that most IH responses occur in the absence of DTH, it has been proposed that the development of IH suppresses DTH responses. However, IgE Ab to Trichophyton antigens are measurable in some subjects with a dual response (Fig. 1) (123). Immediate or delayed skin responses to Trichophyton or antigenic extracts of dermatophytes (trichophytin) are relatively common in the general population. For example, Wood and Cruickshank reported that 30% of uninfected control patients had positive DTH skin tests while 23% had IH skin tests to trichophytin (121). Trichophytin is frequently used to evaluate cell-mediated immunity as part of an anergy panel; however, DTH responses may be absent in healthy individuals, presumably because of a lack of prior infection. There is considerable evidence that DTH is instrumental in the eradication of infection. This comes from (i) the development of DTH in parallel with inflammatory responses in primary infections, (ii) the association between acute highly inflamed lesions and DTH, and (iii) failure to develop infection after experimental inoculation when DTH is present (47, 63-65, 76, 94). Significantly higher titers of specific IgG Ab to purified Trichophyton antigens have been observed among subjects with IH responses compared with individuals with DTH or negative skin tests. Furthermore, both the prevalence and titer of IgG Ab increased significantly with age (123). Given that IH skin tests are often associated with chronic infection, this suggests that the humoral response to Trichophyton is less protective. Although immune mechanisms involved in the natural resolution of infection have yet to be resolved, recent findings suggest that T cells with a defined specificity for fungal antigens are critical to DTH (122, 124).
Immediate Hypersensitivity: a Marker of Immune Status?
In contrast to DTH, IH is frequently associated with chronic noninflammatory dermatophytosis. Indeed, DTH is often absent despite persistent and widespread infection in some individuals. Recently, it has been shown that treatment of chronic dermatophytosis with the systemic antifungal terbinafine can restore DTH responsiveness to intradermal trichophytin antigen (29). In keeping with this, it has been proposed that protracted antigen exposure can induce immunologic unresponsiveness, or anergy, by activating suppressor T cells which then downregulate cell-mediated responses (20). However, several studies have failed to demonstrate diminished T-cell proliferative responses to dermatophyte antigens in patients with IH (51, 66, 74, 124). Alternatively, properties of the fungus itself may prevent the development of cell-mediated responses. For example, mannans derived from T. rubrum have been proposed to inhibit DTH by interfering with antigen-processing pathways required for T-cell activation (20). However, whether chronic infection results from a lack of DTH or from the presence of an IH response is not known. Thus, a major question is whether IH is a prerequisite for the development of persistent infection and, if so, whether it reflects a more broad-based immune dysregulation. Host factors, such as integrity of the skin barrier, play a central role in determining the outcome of dermatophyte infection. For example, chronic dermatophytosis has been reported in association with lamellar ichthyosis, an epidermal barrier defect characterized by thick hyperkeratotic erythematous scaling of the skin (77). The immune status of the host is also relevant. Indeed, it is well recognized that patients with HIV infection or those receiving immunosuppressive therapy are predisposed to develop chronic dermatophytosis and, very rarely, invasive disease (32, 34, 44, 80, 84). This observation is consistent with impairment of cell-mediated immunity associated with these conditions. Changes in the balance between Th1 and Th2 responses have been implicated in the progression of several diseases associated with dermatophytosis, notably HIV infection and diabetes. It is tempting to speculate that Th2 skewing contributes to chronic infection. Indeed, this would be consistent with the view that Th2 polarization contributes to disease progression in HIV-infected subjects (19). However, this does not explain the seemingly paradoxical association between chronic dermatophytosis and diabetes, since the onset of type 1 diabetes is thought to represent a functional shift from Th2- to Th1-type immunity (106). Defining the relationship between diabetes and dermatophytosis is complex, since diabetic complications, including peripheral vascular disease, could contribute to persistent infection. Furthermore, a recent study reported neither increased prevalence of dermatophytosis among diabetic subjects nor an association with complications of diabetes (97). In a case study report, chronic T. rubrum infection associated with IH and a markedly elevated total IgE level in serum was proposed to contribute to the development of severe measles infection by favoring the development of Th2 responses (100). The implication is that a broad-based immune dysregulation could predispose to the progression of distinct disease entities (i.e., of fungal and viral origin). It is tempting to speculate that the increased incidence of onychomycosis in the elderly reflects changes in the immune response; however, analysis of T-cell responses to fungal allergens failed to identify an antigen-specific defect in the T-cell repertoire associated with advanced age (124). The reported association between atopy (i.e., sensitization to allergens) and chronic dermatophytosis has led to the speculation that Th2 responses contribute to persistent infections. However, this relationship, which is discussed in detail below, is highly complex.
ALLERGY AND DERMATOPHYTOSIS
Background: Historical Perspective
Sensitization to allergens, characterized by the development of IH skin tests and circulating allergen-specific IgE Ab, is a major risk factor for the development of asthma. As early as 1949, a high incidence of allergic history in patients with chronic dermatophytosis was reported (48, 61, 63). Although elevated total IgE levels in the serum of patients with chronic dermatophytosis have been reported (7), normal IgE levels have also been observed among individuals with athlete's foot, irrespective of atopic history (87). In 2000, Escalante et al. examined the incidence of dermatophytosis among atopic and nonatopic subjects. Atopy was defined as a positive skin test to mite or cockroach along with a personal or family history of allergic disease. In addition, infection with Trichophyton was confirmed by culture. The results showed no link between dermatophytosis and atopic status, despite an increased prevalence of IH skin tests to Trichophyton in patients with active infection (33). In addition, although significantly increased levels of total IgE in serum were present in atopic compared with nonatopic subjects, the total IgE levels were comparable among nonatopic subjects with and without mycosis. A subsequent study confirmed the association of IH with Trichophyton infection, irrespective of the allergic status of the host (83). Taken together, these findings suggest that dermatophytosis is not associated with atopy per se and that infection does not contribute to elevated total IgE levels. Furthermore, infection, as opposed to atopic status, appears to be the primary determinant of Trichophyton sensitivity.
A recent survey of fungal sensitivity among 3,248 allergic subjects with respiratory symptoms (rhinitis or asthma) showed a 19.1% prevalence of skin prick test reactivity to one of the seven selected fungal genera (Alternaria, Aspergillus, Candida, Cladosporium, Penicillium, Saccharomyces, and Trichophyton) (78). The majority of mold-sensitive subjects exhibited monosensitization, with Alternaria, Candida, or Trichophyton accounting for most cases. Furthermore, 10.2% of subjects who were skin prick test positive to at least one fungus exhibited IH to Trichophyton. There is considerable evidence that, within a minority of allergic subjects, IH to Trichophyton is relevant to the pathogenesis of their allergic disease rather than being a nonspecific corollary of the allergic state. It has been recognized for over a century that inhalation of fungi can induce asthma. A role for dermatophytes in asthma was first described in 1930 (120). In that study, a patient presenting with asthma and severe tinea infections showed improvement in asthma after treatment of the fungal infection (Table 1). Over 30 years later, an association between chronic tinea pedis and chronic urticaria was described (117); this was later substantiated by Platts-Mills et al. (91). In 1980, Jones described the association of allergic rhinitis or asthma with chronic tinea infections, increased total IgE, and Trichophyton-specific IgE levels, and IH as the “atopic-chronic dermatophytosis syndrome” (62). In light of studies which refute the relationship between atopy and chronic infection, this term may be a misnomer. However, more recent studies corroborate Jones' theory that exposure to fungal antigens could induce allergic inflammatory responses in the respiratory tract. Several reports have provided evidence of a role for dermatophyte sensitivity in both asthma and allergic rhinitis (42, 58, 68, 90, 92, 104, 115, 116) (Table 1). In a placebo-controlled challenge study, Ward et al. showed that eight patients with perennial asthma and chronic tinea infection developed bronchial hyperreactivity after nasal challenge with a T. tonsurans extract (115). That study provided the strongest evidence yet of a causal role for dermatophyte antigens in asthma. In support of this, subsequent studies described the improvement of asthma or allergic rhinitis symptoms after oral antifungal therapy (58, 68, 116). Dermatophyte antigens have also been implicated in the pathogenesis of urticaria based on abatement of symptoms after treatment of tinea pedis (117).
TABLE 1.
Chronology of the link between dermatophytosis and allergic disease
| Study (date) (reference) | No. of patients | Allergic disease | Tinea infection | Evidence of specific sensitization | Treatment | Outcome measures | Importance |
|---|---|---|---|---|---|---|---|
| Wise and Sulzberger (1930) (120) | 1 | Perennial asthma and rhinitis | Skin of trunk; history of severe tinea of feet | Elicitation of itch, urticaria; sneeze, and dyspnea after intradermal injection of trichophytin | Local | Clinical response (decreased skin lesions) | First evidence of a causal link between fungal infection and and rhinitis or asthma |
| Weary and Guerrant (1967) (117) | 1 | Chronic urticaria | Skin and nails of feet | IH skin test to trichophytin; flare of urticarial lesions after skin testing | Griseofulvin | Clinical response | Resolution of urticaria after antifungal therapy; relapse after therapy discontinued |
| Platts-Mills et al. (1986) (92) | 1 | Asthmaa | Skin of feet and groin | IH skin test and serum IgE (RAST) to Trichophyton spp/ | Griseofulvin | Clinical response (PEF,c symptoms, steroid use) | First report of “intrinsic” asthma and antifungal therapy |
| Platts-Mills et al. (1987) (91) | 73 | Urticaria, angioedema, asthma or rhinitis | History of skin infection and clinical signs of infection | IH skin tests to Trichophyton spp. 8/27 with urticaria/angioedema, 15/39 with asthma, 2/7 with rhinitis; IgE to Trichophyton spp. (RAST) in 87% of subjects with IH | None | NAd | High prevalence of serum IgE to Trichophyton antigens in patients with IH skin tests and allergic disease |
| Gumowski et al. (1987) (42) | 135 | Asthma or rhinitis with IH skin tests to fungal antigens | NDd | Immediate response to bronchial challenge with C. albicans, Trichophyton, or Epidermophyton (81 patients) | Desensitization (2 yr) | Clinical response and lung function (bronchial challenge) | Evidence of specific sensitization to fungal antigens within the respiratory tract; effect of immunotherapy on bronchial reactivity to Trichophyton |
| Ward et al. (1989) (115) | 10 | Asthma | Skin, toenails, groin | IH skin tests to Trichophyton spp.; serum IgE to T. tonsurans (RAST); immediate response to bronchial challenge with T. tonsurans; positive nasal challenge | None | NAd | Specific reactivity to T. tonsurans in the upper and lower respiratory tract |
| Kivity et al. (1992) (68) | 8 | Perennial rhinitisa | Skin or toenails | IH skin test, serum IgE (RAST), and positive nasal challenge to Trichophyton | Griseofulvin (4 patients) | Clinical response (nasal symptoms) | Effect of antifungal therapy on rhinitis |
| Wilson et al. (1993) (119) | 1 | Atopic dermatitis | Toenails | IH skin test to Trichophyton spp. & Tri t 1; serum IgE to T. tonsurans (RAST); serum IgE & IgG to Tri t 1 (RIA)b | Griseofulvin | Clinical response (skin lesions) | Effect of antifungal therapy on eczema |
| Ward et al. (1999) (116) | 11 | Severe or moderately severe asthma | Toenails (all patients) ± skin | IH skin test to Trichophyton spp.; serum IgE to T. tonsurans (RAST); serum IgE and IgG4 to Tri r 2 or Tri t 4 (RIA); positive bronchial challenge to Trichophyton spp. | Fluconazole | Clinical response (PEF, symptoms, steroid use, bronchial sensitivity) | Placebo-controlled trial of fluconazole |
| Klein et al. (1999) (71) | 1 | Atopic dermatitis: multiple flares with concurrent acute tinea pedis | Skin and nails of feet; positive culture for T. rubrum | IH skin test to Trichophyton spp. | Ketoconazole | Clinical response (skin lesions) | Repeated resolution of atopic dermatitis with clearance of fungal infection |
| Hurlimann and Fah (2001) (58) | 2 | Perennial rhinitis and asthma; chronic dermatitis | Skin and nails of feet; positive culture for T. rubrum | Severe dyspnea after skin prick test with T. rubrum; IH skin test to T. rubrum | Terbinafine | Clinical response (nasal symptoms or skin lesions) | Recurrence of nasal symptoms or skin lesions when treatment discontinued |
Patients exclusively sensitized to Trichophyton.
RIA, antigen binding radioimmunoprecipitation assay.
PEF, peak expiratory flow rate.
ND, not documented; NA, not applicable.
Rajka and Barlinn reported a high incidence (>40%) of IH skin tests to trichophytin in patients with atopic dermatitis (AD) irrespective of infection (93). In that study, trichophytin sensitivity correlated with skin test reactivity to other molds, and it was suggested that this reflected cross-reactivity with other antigens of fungal origin. Indeed, in a recent study of 65 patients with AD, serum IgE ab directed against both inhaled (Alternaria alternata and Aspergillus fumigatus) and colonizing fungi including T. rubrum were present in >50% of subjects (102). Although cross-reactivity has not been excluded as a factor, studies on the effects of antifungal therapy suggest that Trichophyton infection contributes to the pathogenesis of AD, at least in a subset of patients. In 1993, Wilson et al. reported improvement of eczema in an atopic patient with tinea unguium, IH skin tests, and serum IgE Ab to Trichophyton after systemic antifungal therapy (119). In a later report, a patient with AD presented with a history of multiple flares and concurrent acute tinea pedis and onychomycosis. In this patient, systemic antifungal therapy was associated with resolution of infection and improvement in skin condition (71) (Table 1). Similar observations were reported by Kolmer et al. for 5 AD patients who received itraconazole in combination with anti-bacterial therapy (73). However, not all patients in this study were specifically sensitized to Trichophyton. AD is a complex disease which is frequently associated with high titers of IgE Ab to common inhalant allergens, such as dust mite and cat allergens, as well as colonization with a variety of yeasts, most notably Malassezia furfur and C. albicans. Recent studies of AD patients who were cat sensitized identified altered T-cell responses to putative regulatory epitopes of cat allergen (17, 95). Thus, it could be argued that sensitization to Trichophyton in patients with AD reflects a more broad-based immune dysregulation, resulting in sensitization to multiple allergens. Clearly, further studies of AD patients with well-defined patterns of allergen sensitization are warranted.
Trichophyton: an Intrinsic Allergen
Allergens are low-molecular-weight proteins or glycoproteins which induce IgE antibodies in humans. The ability of Trichophyton components to bind IgE Ab has been demonstrated using a variety of techniques including both solid-phase (radioallergosorbent test [RAST], enzyme-linked immunosorbent assay, and Western blot analysis) and fluid-phase (radioimmunoprecipitation) assays (55, 85, 91, 123). As mentioned above, a significant proportion of individuals who are sensitized to Trichophyton show no evidence of sensitization to other allergens, including fungi, as judged by skin prick testing (78). Monosensitivity to Trichophyton can be a feature of asthma presenting in adult life. This type of asthma can be severe (i.e., steroid-dependent) and is often said to be “intrinsic” since it may not be associated with a personal or familial history of allergy to inhalant allergens. Monosensitization to Trichophyton is an important observation since it argues against a role for fungal cross-reactivity in these patients. Intrinsic asthma is thought to arise from colonization of skin or mucosal surfaces by fungal pathogens (Aspergillus or Candida spp.). However, attempts to culture Trichophyton from the nasal passages have yielded negative results (115). Absorption of dermatophyte antigens through the skin, possibly via epidermal barrier defects, is the most likely route of exposure. However, there is some evidence that inhalational exposure can also occur. Podiatrists may be exposed to large quantities of aerosolized nail dust from burring toenails which are infected with dermatophyte fungi. A high prevalence of elevated IgE Ab levels (31% of subjects) and sensitization to Trichophyton (16.5%) have been reported among podiatrists, although the relationship to allergic symptoms is unclear (1, 5, 37).
Ward et al. previously reported 11 patients with severe or moderately severe asthma, all of whom had IH skin tests, serum IgE Ab, and bronchial reactivity to Trichophyton extract (116) (Table 1). Specific IgE and IgG4 Ab to Trichophyton allergens were also present in serum samples obtained from the majority of these patients (Fig. 1) (123, 125). After 5 months of treatment with the systemic antifungal fluconazole, bronchial reactivity to Trichophyton was significantly decreased, as was the requirement for oral steroids. Furthermore, improvement of lung function was maintained for up to 36 months after the start of treatment. It has been argued that the beneficial effects of antifungal treatment could be attributed to other pharmacologic effects that are common to some members of the azole group of drugs, including inhibition of thromboxane synthetase and an inhibitory effect on steroid metabolism. However, no effect on thromboxane synthetase and only weak steroid-inhibitory properties have been reported for fluconazole (10, 21, 23, 127).
Interestingly, many patients who fit the criteria for intrinsic asthma associated with Trichophyton infection exhibit the triad of aspirin sensitivity, nasal polyps, and severe asthma (99). Indeed, in the study carried out by Ward et al., 8 of 11 patients showed evidence of extensive sinus disease as judged by CT scan and 7 of these patients had nasal polyposis (Fig. 1) (116). Furthermore, nasal challenge with Trichophyton extract elicited a marked drop in nasal peak flow, suggesting that Trichophyton sensitivity could also be an etiologic factor for disease processes within the nasal passage. “Aspirin sensitivity” is a broad term used to describe a continuous inflammatory disease of the airways which is accompanied by exacerbations of asthma and rhinitis after ingestion of aspirin. Although mechanisms for this condition remain largely unknown, considerable advances have been made in elucidating the mediators involved. Aspirin exerts its effects by inhibiting cyclooxygenase-1 (COX-1), an enzyme controlling the synthesis of prostaglandin E2. In aspirin sensitivity, inhibition of prostaglandin E2 releases the negative regulatory effects of this mediator on the leukotriene synthesis pathway, leading to unrestrained leukotriene synthesis and subsequent secretion of inflammatory mediators from mast cells and eosinophils. Such events may occur locally, resulting in chronic inflammation of the upper airways (rhinosinusitis and nasal polyps) and lower airways (asthma). Given that the pathogenesis of aspirin sensitivity is not dependent on IgE Ab per se, the relationship between Trichophyton sensitivity and aspirin sensitivity is an enigma. It has been proposed that the respiratory tract is primed for a reaction to aspirin by a variety of triggers which stimulate the migration of inflammatory cells into the mucosa and activation at this site (112). Systemic absorption of dermatophyte antigens through epidermal barrier defects, followed by uptake of antigen by epidermal Langerhans cells and subsequent activation of T cells within regional lymph nodes, could ultimately contribute to inflammatory processes at distal sites (e.g., the upper airways). This may occur via migration of activated T cells or by direct recruitment of antigen-bearing APCs to the upper respiratory tract. Inflammatory responses within the upper airways may then play a key role in triggering reactions in the lower airways. In keeping with this, anecdotal reports of Trichophyton infection preceding the onset of rhinosinusitis are not uncommon (T. A. E. Platts-Mills, personal communication).
Although clinical studies have yielded compelling evidence of a role for Trichophyton in the development of allergic disease, it was not until the 1990s that purified allergens became available. Standard immunochemical techniques and molecular cloning methods resulted in the rapid identification of antigens with potent allergenic properties. This opened the door to studies aimed at defining T-cell responses which govern IH and DTH, as well as identification of putative targets for new therapies.
Trichophyton Allergens: a Role for Enzymatic Function?
In 1990, the first Trichophyton allergen was isolated from an extract of dried T. tonsurans mycelia (25). In that study, an ammonium sulfate-precipitated protein fraction of T. tonsurans extract was subjected to size exclusion chromatography. High IgE binding capacity measured by RAST was detected in an eluted fraction containing low-molecular-mass proteins (12 to 50 kDa). Further purification of this fraction using hydrophobic interaction chromatography yielded a 30-kDa protein. IgE Ab to this T. tonsurans allergen, designated Tri t 1, were measurable in 73% of sera (22 of 30) from subjects with asthma, rhinitis, or urticaria who were sensitized to Trichophyton on the basis of IH skin tests or serum IgE Ab to Trichophyton extract (Table 2). Thus, this protein represented a major allergen. Interestingly, this protein elicited DTH responses in only 1 of 14 subjects with DTH to Trichophyton extract. The authors of that study surmised that DTH responses were attributable to proteins other than Tri t 1. Before that time, most studies had utilized partially purified preparations derived from culture extracts in order to identify antibody binding protein or carbohydrate components. In 1972, Moser and Pollack reported that DTH reactivity was restricted to a glycopeptide fraction derived from Trichophyton and Microsporum spp. which contained mannopeptides (81). In contrast, Barker et al. attributed IH and DTH skin tests to distinct components of T. mentagrophytes consisting of carbohydrates or peptides, respectively (8). In keeping with this latter observation, IgE antibody reactivity to polysaccharide but not peptide antigens derived from T. mentagrophytes was subsequently reported (55). However, it has been proposed that IgE Ab binding to carbohydrate components of fungal extracts may result from nonspecific interactions or the presence of cross-reactive carbohydrate determinants (85). Deuell and colleagues had speculated that DTH responses were directed against a higher-molecular-mass fraction of T. tonsurans (∼70 kDa) (25). In 1996, we reported the purification of an 83-kDa T. tonsurans protein which was designated protein IV (later named Tri t 4 in accordance with standard allergen nomenclature) based on its ability to induce DTH (type IV) skin tests (Table 2) (123). However, this protein also elicited IH skin reactions. Of 10 subjects with DTH responses to Trichophyton extract, 9 had IgG Ab to protein IV but none had measurable IgG4 or IgE. Conversely, IH responses to protein IV were associated with IH skin tests to Trichophyton extract in the majority of subjects and the presence of specific IgE and IgG4 Ab (Fig. 1; Table 2). T-cell lines specific for purified Trichophyton allergens which were established from IH responders produced cytokine profiles characteristic of Th2/Th0 cells (ratio of IFN-γ to IL-4 or IL-5, <2:1). By contrast, T-cell lines from DTH subjects produced cytokines consistent with a Th1 phenotype (ratio of IFN-γ to IL-4 or IL-5, >20:1) (Table 2) (108). These studies established that diverse immune responses (i.e., IH and DTH), which are associated with distinct serologic and cytokine profiles, could be directed against the same protein. Furthermore, they implicated Th1 cells in cell-mediated responses to dermatophyte antigens and suggested that Th2 cells might contribute to infections in subjects with IH.
TABLE 2.
Properties of Trichophyton allergens
| Species | Allergen | Homology | Source: natural or recombinant | Sequencea | Mol mass (kDa) | Immunologic properties | Reference(s) |
|---|---|---|---|---|---|---|---|
| T. tonsurans | Tri t 1 | Exo-1,3-beta-glucanase | Natural | N terminus (30 aa) from protein | 30 | Elicits IH skin tests. Binds serum IgG and IgE Ab. Tri t 1-specific T-cell lines from IH and DTH responders secrete Th0/Th2 and Th1 cytokines, respectively. | 25, 108 |
| Tri t 4 (protein IV) | Serine proteinase (prolyl oligopeptidase [S9] family) | Natural | N-terminus (26 aa) and 9 internal peptides (117 aa) from protein | 83 | Elicits IH and DTH skin tests. Binds serum IgG, IgG4 and IgE Ab. Tri t 4-specific T-cell lines from IH and DTH responders secrete Th0/Th2 & Th1 cytokines, respectively. | 108, 123 | |
| T. rubrum | Tri r 2 | Serine proteinase (class D subfamily of subtilase [S8] enzymes) | Recombinant: putative catalytic domain fused to GSTb (expressed in E. coli) | Full sequence from cDNA (412 aa) includes putative proregion (123 aa) and catalytic domain (289 aa) | 29 | Elicits IH and DTH skin tests. Binds serum IgG and IgE Ab. Stimulates strong in vitro T-cell proliferation and cytokine responses in cultures from IH and DTH responders. DTH-associated major T-cell epitopes induce Th1- and Th2-dominated cytokine profiles in PBMC cultures from DTH responders. | 122, 124, 125 |
| Tri r 4 | Serine proteinase (prolyl oligopeptidase [S9] family) | Recombinant: full-length protein (expressed in Pichia pastoris) | Full sequence from cDNA (726 aa) | 85 | Elicits no skin test reactivity. Shows reduced IgG Ab binding compared with natural Tri t 4. Stimulates weak T-cell proliferation. | 125 |
aa, amino acids.
GST, glutathione S-transferase.
Subsequent work identified the homologue of Tri t 4 (protein IV) derived from T. rubrum using molecular cloning techniques (Table 2). Tri t 4 exhibited amino acid sequence identity to the prolyl oligopeptidase (S9) family of serine proteinases, which is characterized by a catalytic amino acid triad consisting of histidine, aspartic acid, and serine. In addition, a novel T. rubrum allergen was identified (Tri r 2; 29 kDa), which had a high degree of sequence homology to the class D subfamily of subtilase enzymes (Table 2) (125). Both Tri r 2 and Tri r 4 were produced as recombinant proteins by using bacterial (Escherichia coli) and yeast (Pichia pastoris) expression systems, respectively. Recombinant Tri r 2 demonstrated an in vivo biological function based on its ability to elicit DTH responses. Furthermore, a high prevalence of IgE Ab to rTri r 2 was observed in subjects with IH to Trichophyton extract (43%). However, despite sequence homology to known proteinases, enzymatic activity was not detected (rTri r 2) or was only weakly measurable (rTri r 4). Interestingly, although only the first 30 N-terminal amino acid residues of Tri t 1 have been sequenced, this region exhibits high homology to an exo-β-1,3-glucanase (29 kDa) from Saccharomyces cerevisiae (16, 69). Thus, all Trichophyton allergens identified to date are enzyme homologues (Table 2).
The relevance of the biological function of putative Trichophyton enzymes to allergenicity and to disease pathogenesis remains unknown. In relation to Tri t 1, glucanases have been implicated in cell expansion during fungal growth, in cell-cell fusion during mating, and in spore release during sporulation. In addition, glucanases may fulfill an enzymatic function as transglycosylases (16, 86). It is tempting to speculate that, in addition to its allergenic properties, Tri t 1 could contribute to dermatophyte growth by virtue of a biological function. Interestingly, a β-1,3-glucanase derived from natural rubber latex (Hev b 2) also exhibits IgE Ab binding properties (18, 126). Thus, glucanases from diverse sources exhibit allergenic properties. Regarding Tri r 4, some S9 peptidases are involved in a variety of nonenzymatic physiologic processes; for example, the membrane glycoprotein dipeptidyl peptidase IV (DPPIV/CD26) facilitates cell adhesion by interacting with collagen and fibronectin of the extracellular matrix via a cysteine-rich domain (6, 22). DPPIV also processes bioactive peptides implicated in vascular permeability (22). This raises the intriguing possibility that Tri r 4 contributes to disease pathogenesis by exerting similar effects. Analysis of the amino acid sequence of Tri r 2 identified conserved amino acid motifs flanking aspartic acid, histidine, and serine residues which form the catalytic triad characteristic of the subtilase (S8) family of serine proteinases. The highest degree of amino acid sequence identity was between Tri r 2 and proteinase T produced by the thermophilic fungus Tritirachium album (58.2% identity in a 304-amino-acid overlap). Notably, another homologous enzyme was alkaline proteinase (Alp) derived from Aspergillus fumigatus. This enzyme has been proposed as a virulence factor based on decreased suppression of polymorphonuclear leukocyte chemotaxis in mutant strains of A. fumigatus lacking Alp (59). True subtilisins derived from bacteria are among the best characterized of the subtilase enzyme family. Subtilisin Carlsberg (alcalase) produced by Bacillus licheniformis is frequently used in detergent formulations, and allergic respiratory reactions to alcalase have been reported among exposed factory workers (12, 88). More recently, a novel subtilisin homologue with IgE antibody binding properties derived from the fungus Penicillium citrinum was identified (110). This allergen, Pen c 1, has a high degree of amino acid sequence identity to Tri r 2 (J. Woodfolk, unpublished data). This allergen or other cross-reactive allergens derived from molds may play an important role in asthma in regions where sensitization to mold is highly prevalent, such as Taiwan, where more than 70% of asthmatic adults are sensitized to molds (56). Taken together, these observations are consistent with reports of cross-reactivity among different mold genera, as well as among different species derived from the same fungus, e.g., T. rubrum and T. tonsurans.
Although the enzymatic function of Trichophyton allergens has not been analyzed in detail, it remains possible that this is relevant not only to the pathogenesis of dermatophytosis but also to the development of allergic inflammatory responses within the respiratory tract. Many enzyme homologues have now been identified as allergens, and several exhibit enzymatic functions which may be relevant to the development of allergic disease. For example, the dust mite-derived allergen Der p 1 is a cysteine protease which cleaves proteins with potential immunomodulatory effects, including CD23 (the low-affinity IgE receptor) and CD25 (the α subunit of the IL-2 receptor) (54, 103). In addition, this allergen disrupts tight junctions, which may facilitate the transepithelial delivery of allergen in the lungs (114). Despite the identification of Tri r 2 as a member of the subtilase enzyme family, this antigen exhibited no enzymatic activity (125). Subtilases are produced as proenzymes, and the proregion has been proposed to act as a template for correct folding of the carboxyl-terminal domain, which contains the active-site residues (57). Thus, the lack of enzymatic activity of Tri r 2 could have resulted from suboptimal processing of the recombinant protein owing to the absence of the putative proregion. In support of this, computer-assisted molecular modeling of Tri r 2 predicted the same three-dimensional structure as the structurally conserved regions of another subtilase, proteinase K, which exhibits high proteolytic activity (124). Identification of a gene family encoding three subtilisin-like proteinases derived from M. canis has recently been described (24). One of these genes encoded a keratinase that was previously isolated (79). A high sequence identity of each gene to the gene encoding Tri r 2 was described, suggesting a dual role for Tri r 2 in the pathogenesis of infection and in allergic disease.
T-Cell Reactivity to Trichophyton Allergens: Do Distinct Immune Responses Fit the Th1/Th2 Paradigm?
The characterization of distinct CD4+ T-cell subsets in the early 1980s by Mosmann et al. fostered numerous studies on the role of Th1 and Th2 cells in diverse diseases (82). Much evidence has been presented in both murine and human systems to indicate the involvement of different T-helper subsets in the generation of distinct immune responses to the same pathogen. Until recently, the general consensus was that Th1 responses conferred protection while Th2 responses were associated with nonprotective responses and disease progression in certain disease models. Identification of novel Trichophyton allergens with both IH- and DTH-inducing activity provided a unique opportunity for examining whether T cells associated with these responses segregated into distinct subsets. As mentioned above, IH and DTH skin tests to Trichophyton are associated with distinct clinical entities. The triad of chronic dermatophytosis, atopy, and IH to Trichophyton suggests involvement of Th2 cells, while the association between DTH and acute infections points toward a Th1 response.
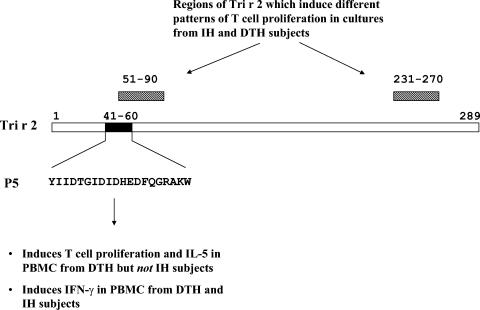
Analysis of T-cell epitopes of Tri r 2 identified differences in the allergen-specific T-cell repertoire among IH and DTH responders. These observations implicated distinct T-cell subsets in mediating different immune responses to the same allergen. Specifically, a peptide mapping to the amino terminus of the molecule, designated peptide 5 (P5), induced strong T-cell proliferation in DTH subjects with diverse HLA types but not in IH responders (124) (Fig. 2). Thus, P5 contained an immunodominant epitope associated with DTH, which could be recognized in a permissive manner. Furthermore, subjects could be classified into IH or DTH groups on the basis of different patterns of T-cell proliferation to two separate regions of Tri r 2 (amino acids 51 to 90 and amino acids 231 to 270) (Fig. 2). Surprisingly, the major DTH-associated epitope, P5, induced IL-5 (along with IL-10) in DTH but not IH subjects. Furthermore, this peptide induced IFN-γ in peripheral blood mononuclear cells derived from both patient groups (122). These observations argue against segregation of Th1 cells and Th2 cells with DTH and IH responses, respectively. Instead, they suggest that Th2 cells are a component of the T-cell repertoire which mediates DTH responses to Tri r 2. Furthermore, T-cell hyporesponsiveness to a specific region of the molecule may be relevant to IH responses. These findings, which conflict with the Th1/Th2 paradigm, are nevertheless consistent with reports of a role for both type 1 and type 2 cytokines in delayed skin reactions associated with Th1-mediated (nickel sensitivity) and Th2-mediated (AD) allergic conditions (41, 67, 109, 118). It has been argued that delayed skin responses induced by Trichophyton do not reflect classical DTH but an alternate response. Indeed, variations in the manifestation of DTH responses in patients with concurrent dermatophytosis have been reported, including contact hypersensitivity to Trichophyton extract applied to the skin and eczematous responses to antigen injected intradermally (113, 121). The picture is further complicated by the presence of a wheal-and-flare reaction which precedes DTH in some individuals.
FIG. 2.
Regions of Tri r 2 which elicit different T-cell responses in IH and DTH subjects. The open box represents the full primary amino acid sequence of the mature form of Tri r 2 (289 amino acids). The solid box corresponds to the amino-terminal major T-cell epitope, P5, consisting of 20 amino acids. The hatched boxes correspond to two regions of the molecule spanned by peptides which induce different patterns of T-cell proliferation in PBMC from IH and DTH subjects.
Regardless, T-cell repertoires which mediate IH and DTH responses exhibit distinct characteristics based on both the pattern of T-cell epitope recognition and cytokine secretion. The question of how such differences could contribute to the pathogenesis of dermatophytosis and of allergic disease remains unanswered. Selective T-cell hyporesponsiveness to specific T-cell antigenic determinants of Tri r 2 (i.e., P5) could reflect changes in the T-cell repertoire which accompany the aging process. This statement is based on the premise that chronic dermatophytosis is frequently observed in older patients. Consistent with this, the mean age of IH responders in our study was markedly higher than that of DTH responders (53 versus 37 years). However, T cells derived from a 74-year-old IH subject showed no change in T-cell epitope-specific proliferation over a 20-month period, suggesting that the allergen-specific T-cell repertoire is stable, even in advanced age. Alternatively, persistent antigen exposure such as that associated with chronic dermatophytosis, occurring over many years, could narrow the T-cell repertoire. In support of this, mice subjected to repeated antigen exposure experience a decrease in diversity of the T-cell repertoire from loss of cells expressing T-cell receptors with lower affinity for antigen (101).
Immune Mechanisms Which Govern the Allergy-Dermatophytosis Relationship
It is perhaps easier to envisage how inhalation of fungally derived allergens, as opposed to percutaneous exposure, could generate allergic responses in the airways. In relation to fungal skin infection, it has been assumed that memory T cells are primed within the draining lymph nodes of the skin and that subsequent T-cell homing to inflammatory sites such as the lungs is a key factor in the allergic inflammatory cascade. Nevertheless, we cannot exclude the possibility that Trichophyton allergens are delivered to the lungs by virtue of their ability to bind to receptors on the surface of APCs. Such receptors may include the high-affinity IgE receptor, FcɛRI, or pattern recognition receptors (Toll-like receptors). By this mechanism, systemic exposure to allergens may facilitate allergen binding to APCs in the blood which preferentially home to sites of allergic inflammation. The plasmacytoid dendritic cell would be one such candidate APC. This cell type induces naive T cells to produce Th2 cytokines and is recruited to the nasal mucosa during experimental allergic rhinitis (60). An important implication is that circulating plasmacytoid dendritic cells can extravasate into peripheral tissues which are devoid of organized lymphoid structures. The same may be true for other types of specialized APCs. Although it has been assumed that allergen capture by dendritic cells occurs at the site of inflammation (i.e., the respiratory tract), “armed” dendritic cells may provide a route of delivery for dermatophyte antigens which gain access through the skin.
In addition to factors which govern the interplay between the allergen and the APC, it has been proposed that properties of dermatophyte antigens could influence migration of T cells to the site of infection. This could then predispose to allergic disease. Differential expression of homing markers on Th1 and Th2 cells has been widely reported. In keeping with this, IL-5-producing Tri r 2-specific cell lines from a subject with chronic dermatophytosis had low expression of the skin-homing marker, cutaneous lymphocyte-associated antigen (CLA). By contrast, tetanus toxoid-stimulated cells from the same individual elaborated Th1 cytokines and expressed higher levels of CLA (77). It was proposed that Th2 cells are less effective than Th1 cells in the immune surveillance of skin (129). Thus, ineffective recruitment of CD4+ T cells into skin lesions could contribute to persistence of infection.
Our observation that DTH-associated major epitopes can induce both Th1- and Th2-dominated responses in DTH subjects suggests that both type 1 and type 2 effector T cells are relevant to resolution of infection. Extending this further, it seems likely that, in patients with Trichophyton asthma, CD4+ T cells which drive allergic inflammatory processes within the lungs are not exclusive to the Th2 subset. Indeed, a role for Th1 responses in murine models of asthma is now emerging (35). Studies which track the trafficking patterns of Trichophyton-specific T cells associated with distinct immune responses could yield valuable insight into immune mechanisms of asthma associated with sensitivity to Trichophyton. Although such studies are hampered by the absence of a lung-specific marker, analysis of CD4+ T cells which express CLA in conjunction with putative Th1 (CXCR3) and Th2 (CCR4) markers may be an important first step.
NEW TECHNIQUES FOR STUDY: PROSPECTS FOR A VACCINE
Although systemic antifungal drugs are efficacious for the eradication of infection and treatment of Trichophyton asthma, there are several disadvantages: (i) treatment is long-term, often lasting from several months to several years; (ii) recurrence of infection is common once therapy is discontinued; and (iii) long-term therapy with some azoles is associated with side effects. Thus, the development of new treatments is warranted. Recent technologic advances have the potential to identify novel Trichophyton antigens with a dual role in disease pathogenesis and allergy. Such antigens could provide novel targets for therapy of chronic infection associated with allergy. To date, very few allergens derived from Trichophyton have been identified. Initial characterization of Trichophyton allergens by standard immunochemical techniques was challenging. Although the development of recombinant DNA technology in the 1990s facilitated the subsequent identification of additional allergens, it seems likely that other allergens remain to be identified.
The production of hygromycin B-resistant transformants of T. mentagrophytes in the late 1980s demonstrated the feasibility of molecular genetic studies of Trichophyton (40). Generation of deletion mutants of fungal species which lack expression of specific genes is a well-established method. Analysis of Trichophyton species which lack expression of Tri r 2 and Tri r 4, or their homologues, could yield insight into the importance of these antigens as targets for both T-cell responses and B-cell (i.e., IgE and IgG) responses, as well as into their biological function in vivo. In relation to this latter point, a skin explant model has recently been developed which may mimic dermatophyte infection of human skin (26). Skin of full epidermis thickness which was obtained from surgical specimens was inoculated with T. mentagrophytes spores, and infection was confirmed by electron microscopy. This model has the potential to elucidate the role of specific antigens in disease pathogenesis by testing the ability of deletion mutants to establish infection and analyzing gene expression at different stages of the disease process.
Development of a vaccine which has the ability to induce a strong protective memory T-cell response to Trichophyton could provide an advantage over existing treatments. However, identifying the pertinent molecular target for therapy poses a major challenge. As with any pathogen, extracellular proteins are strong candidates. Genetic techniques which selectively identify secreted genes could facilitate the characterization of these antigens from Trichophyton (70). Vaccines incorporating peptides which preferentially stimulate T-cell responses to DTH-associated epitopes may also be effective for the eradication of disease. With this in mind, definition of immune mechanisms which govern distinct immune responses to Trichophyton may be pivotal to understanding the host determinants of protective immunity.
CONCLUSIONS
There is a considerable body of evidence to support the link between dermatophytosis and allergic diseases such as asthma, urticaria, and atopic dermatitis. Although dermatophytosis contributes to symptoms among only a minority of subjects with chronic skin infections, allergic disease can be severe in these patients, often requiring long-term antifungal therapy. In contrast to IH responses, DTH to Trichophyton appears to be protective based on (i) the association with acute as opposed to chronic infection, (ii) the absence of allergen-specific IgG4 or IgE Ab, and (iii) lack of any reports of associated systemic disease. Characterization of the molecular and immunologic properties of Trichophyton allergens has provided insight into the immune mechanisms which govern dichotomous immune responses to Trichophyton. Identification of major T-cell epitopes specific to DTH provides an avenue for the design of new treatments. Trichophyton allergens appear to be important targets for the immune response in both sensitized (IH) and nonsensitized (DTH) subjects. Characterization of additional allergens which contribute to the pathogenesis of Trichophyton infection could have broad implications for the treatment of dermatophytosis in the general population.
Acknowledgments
This work was supported by NIH grants AI-50989 and AI-20565.
I am grateful to Thomas Platts-Mills for helpful comments on the manuscript.
REFERENCES
- 1.Abramson, C., and J. Wilton. 1992. Nail dust aerosols from onychomycotic toenails. II. Clinical and serologic aspects. 1984. J. Am. Podiatr. Med. Assoc. 82:116-123. [DOI] [PubMed] [Google Scholar]
- 2.Adams, B. B. 2000. Tinea corporis gladiatorum: a cross-sectional study. J. Am. Acad. Dermatol. 43:1039-1041. [DOI] [PubMed] [Google Scholar]
- 3.Adams, B. B. 2002. Dermatologic disorders of the athlete. Sports Med. 32:309-321. [DOI] [PubMed] [Google Scholar]
- 4.Alenius, H., N. Kalkkinen, M. Lukka, T. Reunala, K. Turjanmaa, S. Makinen-Kiljunen, E. Yip, and T. Palosuo. 1995. Prohevein from the rubber tree (Hevea brasiliensis) is a major latex allergen. Clin. Exp. Allergy 25:659-665. [DOI] [PubMed] [Google Scholar]
- 5.Alonso, A., C. H. Pionettri, K. Mouchian, J. F. Albonico, S. G. Iraneta, M. Potenza, and C. Iovannitti. 2003. Hypersensitivity to Trichophyton rubrum antigens in atopic and non-atopic podiatrists. Allergol. Immunopathol. 31:70-76. [DOI] [PubMed] [Google Scholar]
- 5a.Anonymous. 1979. Prevalence, morbidity, and cost of dermatological diseases. J. Investig. Dermatol. 73:395-401. [DOI] [PubMed] [Google Scholar]
- 6.Antczak, C., I. DeMeester, and B. Bauvois. 2001. Ectopeptidases in pathophysiology. Bioessays 23:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balogh, E., E. Forizs, M. Debreczeni, and M. Szabolcsy. 1981. Serum IgE level and T-cell count in chronic dermatophytosis. Mykosen 24:84-89. [DOI] [PubMed] [Google Scholar]
- 8.Barker, S. A., C. N. D. Cruickshank, J. H. Morris, and S. R. Wood. 1962. The isolation of trichophytin glycopeptide and its structure in relationship to the immediate and delayed hypersensitivity reactions. Immunology 5:627-632. [PMC free article] [PubMed] [Google Scholar]
- 9.Becerril-Chihu, G., E. Bazan-Mora, R. Lopez-Martinez, C. Sosa-de-Martinez, and R. Ruiz-Maldonado. 1999. How often are dermatophytes present in apparently normal versus scaly feet of children? Pediatr. Dermatol. 16:87-89. [DOI] [PubMed] [Google Scholar]
- 10.Beentens, J. R., W. Loots, Y. Somers, M. C. Coene, and F. DeClerck. 1986. Ketoconazole inhibits the biosynthesis of leukotrienes in vitro and in vivo. Biochem. Pharmacol. 35:883-891. [DOI] [PubMed] [Google Scholar]
- 11.Beller, M., and B. D. Gessner. 1994. An outbreak of tinea corporis gladiatorum on a high school wrestling team. J. Am. Acad. Dermatol. 31:197-201. [DOI] [PubMed] [Google Scholar]
- 12.Biagini, R. E., R. J. Driscoll, D. I. Bernstein, T. G. Wilcox, G. M. Henningsen, B. A. MacKenzie, G. A. Burr, J. D. Scinto, and E. S. Baumgardner. 1996. Hypersensitivity reactions and specific antibodies in workers exposed to industrial enzymes at a biotechnology plant. J. Appl. Toxicol. 16:139-145. [DOI] [PubMed] [Google Scholar]
- 13.Bokhari, M. A., I. Hussain, M. Jahangir, T. S. Haroon, S. Aman, and K. Khurshid. 1999. Onychomycosis in Lahore, Pakistan. Int. J. Dermatol. 38:591-595. [DOI] [PubMed] [Google Scholar]
- 14.Brilhante, R. S., G. C. Paixao, L. K. Salvion, M. J. Diogenes, S. P. Bandeira, M. F. Roch, J. B. Santos, and J. J. Sidrim. 2000. Epidemiology and ecology of dermatophytoses in the city of Fortaleza: Trichophyton tonsurans as important emerging pathogen of tinea capitis. Rev. Soc. Bras. Med. Trop. 33:417-425. [DOI] [PubMed] [Google Scholar]
- 15.Burzykowski, T., G. Molenberghs, D. Abeck, E. Haneke, R. Hay, A. Katsamba, D. Roseeuw, P Van De Kerkhof, R. Van Aelst, and G. Marynissen. 2003. High prevalence of foot diseases in Europe: results of the Achilles Project. Mycoses 46:496-505. [DOI] [PubMed] [Google Scholar]
- 16.Cappellaro, C., V. Mrsa, and W. Tanner. 1998. New potential cell wall glucanases of Saccharomyces cerevisiae and their involvement in mating. J. Bacteriol. 180:5030-5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carneiro R., A. Reefer, B. Wilson, T. Platts-Mills, N. Custis, and J. Woodfolk. 2004. T-cell epitope-specific defects in the immune response to cat allergen in patients with atopic dermatitis. J. Investig. Dermatol. 122:927-936. [DOI] [PubMed] [Google Scholar]
- 18.Chye, M. L., and K. Y. Cheung. 1995. Beta-1,3-glucanase is highly expressed in laticifers of Hevea brasiliensis. Plant Mol. Biol. 29:397-402. [DOI] [PubMed] [Google Scholar]
- 19.Clerici, M., and G. M. Shearer. 1993. A Th1 to Th2 switch is a critical step in the etiology of HIV infection. Immunol. Today 14:107-111. [DOI] [PubMed] [Google Scholar]
- 20.Dahl, M. V., and S. A. Grando. 1994. Chronic dermatophytosis: what is special about Trichophyton rubrum? Adv. Dermatol. 9:97-109. [PubMed] [Google Scholar]
- 21.De Coster, R., W. Wouters, and J. Bruynseels. 1996. P450-dependent enzymes as targets for prostate cancer therapy. J. Steroid Biochem. Mol. Biol. 56:133-143. [DOI] [PubMed] [Google Scholar]
- 22.DeMeester, I., S. Korom, J. Van Damme, and S. Scharpe. 1999. CD26, let it cut or cut it down. Immunol. Today 20:367-375. [DOI] [PubMed] [Google Scholar]
- 23.Denner, K., R. Vogel, W. Schmalix, J. Doehmer, and R. Bernhardt. 1995. Cloning and stable expression of the human mitochondrial cytochrome P45011B1 cDNA in V79 Chinese hamster cells and their application for testing of potential inhibitors. Pharmacogenetics 5:89-96. [DOI] [PubMed] [Google Scholar]
- 24.Descamps, F., F. Brouta, M. Monod, C. Zaugg, D. Baar, B. Losson, and B. Mignon. 2002. Isolation of a Microsporum canis gene family encoding three subtilisin-like proteases expressed in vivo. J. Investig. Dermatol. 119:830-835. [DOI] [PubMed] [Google Scholar]
- 25.Deuell, B., L. K. Arruda, M. L. Hayden, M. D. Chapman, and T. A. E. Platts-Mills. 1991. Trichophyton tonsurans allergen I: characterization of a protein that causes immediate but not delayed hypersensitivity. J. Immunol. 147:96-101. [PubMed] [Google Scholar]
- 26.Duek, L., G. Kaufman, Y. Ulman, and I. Berdicevsky. 2004. The pathogenesis of dermatophyte infections in human skin sections. J. Infect. 48:175-180. [DOI] [PubMed] [Google Scholar]
- 27.Elewski, B. E. 1998. Onychomycosis: pathogenesis, diagnosis, and management. Clin. Microbiol. Rev. 11:415-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elewski, B. E., and M. A. Charif. 1997. Prevalence of onychomycosis in patients attending a dermatology clinic in northeastern Ohio for other conditions. Arch. Dermatol. 133:1172-1173. (Letter.) [PubMed] [Google Scholar]
- 29.Elewski, B. E., M. El Charif, K. D. Cooper, M. Ghannoum, and J. E. Birnbaum. 2002. Reactivity to trichophytin antigen in patients with onychomycosis: effect of terbinafine. J. Am. Acad. Dermatol. 46:371-375. [DOI] [PubMed] [Google Scholar]
- 30.Ellabib, M. S., M. Agaj, Z. Khalifa, and K. Kavanagh. 2002. Yeasts of the genus Candida are the dominant cause of onychomycosis in Libyan women but not men: results of a 2-year surveillance study. Br. J. Dermatol. 146:1038-1041. [DOI] [PubMed] [Google Scholar]
- 31.Ellis, D. H., A. B. Watson, J. E. Marley, and T. G. Williams. 1997. Non-dermatophytes in onychomycosis of the toenails. Br. J. Dermatol. 136:490-493. [PubMed] [Google Scholar]
- 32.Erbagci, Z. 2002. Deep dermatophytoses in association with atopy and diabetes mellitus: Majocchi's granuloma trichophyticum or dermatophyticum pseudomycetoma? Mycopathologia 154:163-169. [DOI] [PubMed] [Google Scholar]
- 33.Escalante, M. T., M. Sanchez-Borges, A. Capriles-Hulett, E. Belfort, E. Di Biagio, and L. Gonzalez-Aveledo. 2000. Trichophyton-specific IgE in patients with dermatophytosis is not associated with aeroallergen sensitivity. J. Allergy Clin. Immunol. 105:547-551. [DOI] [PubMed] [Google Scholar]
- 34.Faergemann, J., and R. Baran. 2003. Epidemiology, clinical presentation and diagnosis of onychomycosis. Br. J. Dermatol. 149(Suppl. 65):1-4. [DOI] [PubMed] [Google Scholar]
- 35.Fulkerson, P. C., N. Zimmermann, E. B. Brandt, E. E. Muntel, M. P. Doepker, J. L. Kavanaugh, A. Mishra, D. P. Wityte, H. Zhang, J. M. Farber, M. Yang, P. S. Foster, and M. E. Rothenberg. 2004. Negative regulation of eosinophil recruitment to the lung by the chemokine monokine induced by IFN-γ. Proc. Natl. Acad. Sci. USA 101: 1987-1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fuller, L. C., F. C. Child, G. Midgley, and E. M. Higgins. 2003. Scalp ringworm in south-east London and an analysis of a cohort of patients from a paediatric dermatology department. Br. J. Dermatol. 148:985-988. [DOI] [PubMed] [Google Scholar]
- 37.Gatley, M. 1991. Human nail dust: hazard to chiropodists or merely nuisance? J. Soc. Occup. Med. 41:121-125. [DOI] [PubMed] [Google Scholar]
- 38.Ghannoum, M. A., R. A. Haijeh, R. Scher, N. Konnikov, A. K. Gupta, R. Summerbell, S. Sullivan, R. Daniel, P. Krusinski, P. Fleckman, . Rich, R. Odom, R. Aly, D. Pariser, M. Zaiac, G. Rebell, J. Lesher, B. Gerlach., G. F. Ponce-de-Leon, A. Ghannoum, J. Warner, N. Isham, and B. Elewski. 2000. A large-scale North American study of fungal isolates from nails: the frequency of onychomycosis, fungal distribution, and antifungal susceptibility patterns. J. Am. Acad. Dermatol. 43:641-648. [DOI] [PubMed] [Google Scholar]
- 39.Ghannoum, M., N. Isham, R. Hajjeh, M. Cano, F. Al-Hawasi, D. Yearick, J. Warner, L. Long, C. Jessup, and B. Elewski. 2003. Tinea capitis in Cleveland: survey of elementary school students. J. Am. Acad. Dermatol. 48:189-193. [DOI] [PubMed] [Google Scholar]
- 40.Gonzalez, R., S. Ferrer, J. Buesa, and D. Ramon. 1989. Transformation of the dermatophyte Trichophyton mentagrophytes to hygromycin B resistance. Infect. Immun. 57:2923-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grewe, M., K. Gyufko, K. Schopf, J. Krutmann. 1994. Lesional expression of interferon-γ in atopic eczema. Lancet 343:25-26. [DOI] [PubMed] [Google Scholar]
- 42.Gumowski, P., B. Lech, I. Chaves, and J. P. Girard. 1987. Chronic asthma and rhinitis due to Candida albicans, Epidermophyton, and Trichophyton. Ann. Allergy 59:48-51. [PubMed] [Google Scholar]
- 43.Gupta, A. K., H. C. Jain, C. E. Lynde, G. N. Watteel, and R. C. Summerbell. 1997. Prevalence and epidemiology of unsuspected onychomycosis in patients visiting dermatologists' offices in Ontario, Canada: a multicenter survey of 2001 patients. Int. J. Dermatol. 36:783-787. [DOI] [PubMed] [Google Scholar]
- 44.Gupta, A. K., N. Konnikov, P. MacDonald, P. Rich, N. W. Rodger, M. W. Edmonds, R. McManus, and R. C. Summerbell. 1998. Prevalence and epidemiology of toenail onychomycosis in diabetic subjects: a multicentre survey. Br. J. Dermatol. 139:665-671. [DOI] [PubMed] [Google Scholar]
- 45.Gupta, A. K., R. G. Sibbald, C. W. Lynde, P. R. Hull, R. Prussick, N. H. Shear, P. De Doncker, C. R. Daniell III, and B. E. Elewski. 1997. Onychomycosis in children: prevalence and treatment strategies. J. Am. Acad. Dermatol. 36:395-402. [DOI] [PubMed] [Google Scholar]
- 46.Gupta, A. K., and R. C. Summerbell. 1998. Increased incidence of Trichophyton tonsurans tinea capitis in Ontario, Canada between 1985 and 1996. Med. Mycol. 36:55-60. [PubMed] [Google Scholar]
- 47.Hanifin, J. M., L. F. Ray, and W. C. Lobitz. 1974. Immunological reactivity in dermatophytosis. Br. J. Dermatol. 90:1-8. [PubMed] [Google Scholar]
- 48.Hay, R. J. 1982. Chronic dermatophyte infections. I. Clinical and mycological features. Br. J. Dermatol. 106:1-7. [DOI] [PubMed] [Google Scholar]
- 49.Hay, R. J. 2001. The future of onychomycosis therapy may involve a combination of approaches. Br. J. Dermatol. 145(Suppl. 60):3-8. [PubMed] [Google Scholar]
- 50.Hay, R. J., W. Robles, G. Midgley, and M. K. Moore for the European Confederation of Medical Mycology Working Party on Tinea Capitis. 2001. Tinea capitis in Europe: new perspective on an old problem. J. Eur. Acad. Dermatol. Venereol. 15:229-233. [DOI] [PubMed] [Google Scholar]
- 51.Hay, R. J. and G. Shennan. 1982. Chronic dermatophyte infections. II. Antibody and cell-mediated immune responses. Br. J. Dermatol. 106:191-198. [DOI] [PubMed] [Google Scholar]
- 52.Hazen, P. G., and M. L. Weil. 1997. Itraconazole in the prevention and management of dermatophytosis in competitive wrestlers. J. Am. Acad. Dermatol. 36:481-482. [DOI] [PubMed] [Google Scholar]
- 53.Heikkila, H., and S. Stubb. 1995. The prevalence of onychomycosis in Finland. Br. J. Dermatol. 133:699-703. [DOI] [PubMed] [Google Scholar]
- 54.Hewitt, C. R. A., A. P. Brown, B. J. Hart, and D. I. Pritchard. 1995. A major house dust mite allergen disrupts the immunoglobulin E network by selectively cleaving CD23: innate protection by antiproteases. J. Exp. Med. 182:1537-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Honbo, S., H. E. Jones, and W. M. Artis. 1984. Chronic dermatophyte infection: evaluation of the Ig class-specific antibody response reactive with polysaccharide and peptide antigens derived from Trichophyton mentagrophytes. J. Investig. Dermatol. 82:287-290. [DOI] [PubMed] [Google Scholar]
- 56.Hsieh, K. H., and J. J. Shen. 1988. Prevalence of childhood asthma in Taipei, Taiwan, and other Asian Pacific countries. J. Asthma 25:73-82. [DOI] [PubMed] [Google Scholar]
- 57.Hu, Z., K. Haghjoo, and F. Jordan. 1996. Further evidence for the structure of the subtilisin propeptide and for its interactions with mature subtilisin. J. Biol. Chem. 271:3375-3384. [DOI] [PubMed] [Google Scholar]
- 58.Hurlimann, A. and J. Fah. 2001. Asthma, rhinitis and dermatitis triggered by fungal infection: therapeutic effects of terbinafine. Dermatology 202:330-332. [DOI] [PubMed] [Google Scholar]
- 59.Ikegami, Y., R. Amitani, T. Murayam, R. Nawada, W. J. Lee, R. Kawanami, and F. Kuze. 1998. Effects of alkaline protease or restrictocin deficient mutants of Aspergillus fumigatus on human polymorphonuclear leukocytes. Eur. Respir. J. 12:607-611. [DOI] [PubMed] [Google Scholar]
- 60.Jahnsen, F. L., F. L. Johansen, J. F. Dunne, L. Farkas, R. Haye, and P. Brandtzaeg. 2000. Experimentally induced recruitment of plasmacytoid (CD123high) dendritic cells in human nasal allergy. J. Immunol. 165:4062-4068. [DOI] [PubMed] [Google Scholar]
- 61.Jillson, O. F., and M. Huppert. 1949. The immediate wheal and 24-48 hour tuberculin type edematous reactions to trichophytin. J. Investig. Dermatol. 12:179-185. [PubMed] [Google Scholar]
- 62.Jones, H. E. 1980. The atopic-dermatophytosis syndrome. Acta Dermatovener. (Stockholm) Suppl. 92:81-85. [Google Scholar]
- 63.Jones, H. E., J. H. Reinhardt, and M. G. Rinaldi. 1973. A clinical, mycological, and immunological survey for dermatophytosis. Arch. Dermatol. 108:61-65. [PubMed] [Google Scholar]
- 64.Jones, H. E., J. H. Reinhardt, and M. G. Rinaldi. 1974. Model dermatophytosis in naturally-infected subjects. Arch. Dermatol. 110:369-374. [PubMed] [Google Scholar]
- 65.Kaaman, T. 1981. Cell-mediated reactivity in dermatophytosis: differences in skin responses to purified trichophytin in tinea pedis and tinea cruris. Acta Derm. Venereol. 61:119-123. [PubMed] [Google Scholar]
- 66.Kaaman, T., B. Petrini, and S. Wasserman. 1979. In vivo and in vitro immune responses to trichophytin in dermatophytosis. Acta Derm. Venereol. 59:229-233. [PubMed] [Google Scholar]
- 67.Kapsenberg, M. L., E. A. Wierenga, F. E. M. Stiekema, A. B. Tiggelman, and J. D. Bos. 1992. Th1 lymphokine production profiles of nickel-specific CD4+ T lymphocyte clones from nickel contact allergic and nonallergic individuals. J. Investig. Dermatol. 98:59-63. [DOI] [PubMed] [Google Scholar]
- 68.Kivity, S., Y. Schwarz, and E. Fireman. 1992. The association of perennial rhinitis with Trichophyton infection. Clin. Exp. Allergy 22:498-500. [DOI] [PubMed] [Google Scholar]
- 69.Klebl, F., and W. Tanner. 1989. Molecular cloning of a cell wall exo-beta-1,3-glucanase from Saccaromyces cerevisiae. J. Bacteriol. 171:6259-6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Klein, R. D., Q. Gu, A. Goddard, and A. Rosenthal. 1996. Selection for genes encoding secreted proteins and receptors. Proc. Natl. Acad. Sci. USA 93:7108-7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Klein, P. A., R. A. Clark, N. H. Nicol. 1999. Acute infection with Trichophyton rubrum associated with flares of atopic dermatitis. Cutis 63:171-172. [PubMed] [Google Scholar]
- 72.Kohl, T. D., and M. Lisney. 2000. Tinea gladiatorum. Sports Med. 29:439-447. [DOI] [PubMed] [Google Scholar]
- 73.Kolmer, H., E. Taketomi, K. Hazen, E. Hughs, B. Wilson, and T. Platts-Mills. 1996. Effect of combined antibacterial and antifungal treatment in severe atopic dermatitis. J. Allergy Clin. Immunol. 98:702-707. [DOI] [PubMed] [Google Scholar]
- 74.Leibovici, V., R. Evron, O. Axelrod, M. Westerman, M. Shalit, V. Barak, and S. Frankenburg. 1995. Imbalance of immune responses in patients with chronic and widespread fungal skin infection. Clin. Exp. Dermatol. 20:390-394. [DOI] [PubMed] [Google Scholar]
- 75.Lein, P. A., R. A. Clark, and N. H. Nicol. 1999. Acute infection with Trichophyton rubrum associated with flares of atopic dermatitis. Cutis 63:171-172. [PubMed] [Google Scholar]
- 76.Lepper, A. W. D. 1974. Experimental bovine Trichophyton verrucosum infection. The cellular responses in primary lesions of the skin resulting from surface or intradermal inoculation. Res. Vet. Sci. 16:287-298. [PubMed] [Google Scholar]
- 77.Ludwig, R. J., J. A. Woodfolk, M. Grundmann-Kollmann, R. Enzensberger, U. Runne, T. A. Platts-Mills, R. Kaufmann, and T. M. Zollner. 2001. Chronic dermatophytosis in lamellar ichthyosis: relevance of a T-helper 2-type immune response to Trichophyton rubrum. Br. J. Dermatol. 145:518-521. [DOI] [PubMed] [Google Scholar]
- 78.Mari, A., P. Schneider, V. Wally, M. Breitenbach, and B. Siomon-Nobbe. 2003. Sensitization to fungi: epidemiology, comparative skin tests, and IgE reactivity of fungal extracts. Clin. Exp. Allergy 33:1429-1438. [DOI] [PubMed] [Google Scholar]
- 79.Mignon, B., M. Swinnen, J. P. Bouchara, M. Hofinger, A. Nikkels, G. Pierard, C. Gerday, and B. Losson. 1998. Purification and characterization of a 31.5 kDa keratinolytic subtilisin-like serine protease from Microsporum canis and evidence of its secretion in naturally infected cats. Med. Mycol. 36:395-404. [PubMed] [Google Scholar]
- 80.Mirmirani, P., N. A. Hessol, T. A. Maurer, T. G. Berger, P. Nguyen, A. Khalsa, A. Gurtman, S. Micci, M. Young, S. Homan, S. J. Gange, and R. M. Greenblatt. 2001. Prevalence and predictors of skin disease in the Women's Interagency HIV Study (WIHS). J. Am. Acad. Dermatol. 44:785-788. [DOI] [PubMed] [Google Scholar]
- 81.Moser, S. A., and J. D. Pollack. 1978. Isolation of glycopeptides with skin test activity from dermatophytes. Infect. Immun. 19:1031-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mosmann, T. R., H. Cherwinski, M. W. Bond, M. A. Giedlin, and R. L. Coffman. 1986. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 136:2348-2357. [PubMed] [Google Scholar]
- 83.Mungan, D., S. Bavbek, Y. Peksari, G. Celik, E. Gurgey, Z. Misirligil. 2001. Trichophyton sensitivity in allergic and nonallergic asthma. Allergy 56:558-562. [DOI] [PubMed] [Google Scholar]
- 84.Nir-Paz, R., H. Elinav, G. E. Pierard, D. Walker, A. Maly, M. Shapiro, R. C. Barton, and I. Polacheck. 2003. Deep infection by Trichophyton rubrum in an immunocompromised patient. J. Clin. Microbiol. 41:5298-5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nissen, D., L. J. Petersen, R. Esch, E. Svejgaard, P. S. Skov, L. K. Poulsen, and H. Nolte. 1998. IgE-sensitization to cellular and culture filtrates of fungal extracts in patients with atopic dermatitis. Ann. Allergy Asthma Immunol. 81:247-255. [DOI] [PubMed] [Google Scholar]
- 86.Norbeck, J., and A. Blomberg. 1996. Protein expression during exponential growth in 0.7 M NaCl medium of Saccharomyces cerevisiae. FEMS Microbiol. Lett. 137:1-8. [DOI] [PubMed] [Google Scholar]
- 87.O'Loughlin, S., J. L. Diaz-Perez, G. J. Gleich, and R. K. Winkelmann. 1977. Serum IgE in dermatitis and dermatosis: an analysis of 497 cases. Arch. Dermatol. 113:309-315. [PubMed] [Google Scholar]
- 88.Pepys, J., I. D. Wells, M. F. D'Souza, and M. Greenberg. 1973. Clinical and immunological responses to enzymes of Bacillus subtilis in factory workers and consumers. Clin. Allergy 3:143-160. [DOI] [PubMed] [Google Scholar]
- 89.Perea, S., M. J. Ramos, M. Garau, A. Gonzalez, A. R. Noriega, and A. del Palacio. 2000. Prevalence and risk factors of tinea unguium and tinea pedis in the general population in Spain. J. Clin. Microbiol. 38:3226-3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Platts-Mills, T. A. E., R. S. Call, B. A. Deuell, G. Karlsson, and G. W. Ward. 1992. The association of hypersensitivity diseases with dermatophyte infections. Clin. Exp. Allergy 22:427-428. [DOI] [PubMed] [Google Scholar]
- 91.Platts-Mills, T. A. E., G. P. Fiocco, M. L. Hayden, J. L. Guerrant, S. M. Pollart, and S. R. Wilkins. 1987. Serum IgE antibodies to Trichophyton in patients with urticaria, angioedema, asthma, and rhinitis: development of a radioallergosorbent test. J. Allergy Clin. Immunol. 79:40-45. [DOI] [PubMed] [Google Scholar]
- 92.Platts-Mills, T. A. E., G. P. Fiocco, S. M. Pollart, M. L. Hayden, S. Jackson, and S. R. Wilkins. 1986. Trichophyton allergy in a 24-year-old man with intrinsic asthma. Ann. Allergy 56:454-455. [PubMed] [Google Scholar]
- 93.Rajka, G., and C. Barlinn. 1979. On the significance of the trichophytin reactivity in atopic dermatitis. Acta Derm. Venereol. 59:45-47. [PubMed] [Google Scholar]
- 94.Rasmussen, J. E., and A. R. Ahmed. 1978. Trichophytin reactions in children with tinea capitis. Arch. Dermatol. 114:371-372. [PubMed] [Google Scholar]
- 95.Reefer, A. J., R. M. Carneiro, N. J. Custis, T. A. E. Platts-Mills, S. J. Sung, J. Hammer, and J. A. Woodfolk. 2004. A role for IL-10-mediated HLA-DR7-restricted T-cell dependent events in development of the modified Th2 response to cat allergen. J. Immunol. 172:2763-2772. [DOI] [PubMed] [Google Scholar]
- 96.Roberts, D. T. 1992. Prevalence of dermatophyte onychomycosis in the United Kingdom: results of an omnibus survey. Br. J. Dermatol. 126(Suppl. 39):23-27. [DOI] [PubMed] [Google Scholar]
- 97.Romano, C., L. Massai, F. Asta, and A. M. Signorini. 2001. Prevalence of dermatophytic skin and nail infections in diabetic patients. Mycoses 44:83-86. [DOI] [PubMed] [Google Scholar]
- 98.Sais, G., A. Juggla, and J. Peyri. 1995. Prevalence of dermatophyte onychomycosis in Spain: a cross-sectional study. Br. J. Dermatol. 132:758-761. [DOI] [PubMed] [Google Scholar]
- 99.Samter, M., and R. F. Beers, Jr. 1968. Intolerance to aspirin: clinical studies and consideration of its pathogenesis. Ann. Intern. Med. 68:975-983. [DOI] [PubMed] [Google Scholar]
- 100.Sato, N, and H. Tagami. 2003. Severe measles in a young female patient with chronic, generalized Trichophyton rubrum infection showing type 2 helper T cell-dominant immunologic reactivity. J. Am. Acad. Dermatol. 48:S43-S46. [DOI] [PubMed] [Google Scholar]
- 101.Savage, P. A., J. J. Boniface, and M. M. Davis. 1999. A kinetic basis for T cell receptor repertoire selection during an immune response. Immunity 10:485-492. [DOI] [PubMed] [Google Scholar]
- 102.Scalabrin, D. M. F., S. Bavbek, M. S. Perzanowski, B. B. Wilson, T. A. E. Platts-Mills, and L. M. Wheatley. 1999. Use of specific IgE in assessing the relevance of fungal and dust mite allergens to atopic dermatitis: a comparison with asthmatic and nonasthmatic control subjects. J. Allergy Clin. Immunol. 104:1273-1279. [DOI] [PubMed] [Google Scholar]
- 103.Schulz, O., H. F. Sewell, and F. Shakib. 1998. Proteolytic cleavage of CD25, the alpha subunit of the human T cell interleukin 2 receptor, by Der p 1, a major mite allergen with cysteine protease activity. J. Exp. Med. 187:271-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schwartz, H. J., and G. W. Ward. 1995. Onychomycosis, Trichophyton allergy and asthma—a causal relationship? Ann. Allergy Asthma Immunol. 74:523-524. [PubMed] [Google Scholar]
- 105.Seebacher, R. 2003. The change of dermatophyte spectrum in dermatomycoses. Mycoses 46 (Suppl. 1):42-46. [PubMed] [Google Scholar]
- 106.Shimada, A., B. Charlton, P. Rohane, C. Taylor-Edwards, and C. G. Fathman. 1996. Immune regulation in type 1 diabetes. J. Autoimmun. 9:263-269. [DOI] [PubMed] [Google Scholar]
- 107.Singh, D., D. C. Patel, K. Rogers, N. Wood, D. Riley, and A. J. Morris. 2003. Epidemiology of dermatophyte infection in Auckland, New Zealand. Australas. J. Dermatol. 44:263-266. [DOI] [PubMed] [Google Scholar]
- 108.Slunt, J. B., E. A. Taketomi, J. A. Woodfolk, M. L. Hayden, and T. A. E. Platts-Mills. 1996. The immune response to Trichophyton tonsurans: distinct T cell cytokine profiles to a single protein among subjects with immediate and delayed hypersensitivity. J. Immunol. 157:5192-5197. [PubMed] [Google Scholar]
- 109.Spergel, J. M., E. Mizoguchi, H. Oettgen, A. K. Bhan, and R. S. Geha. 1999. Roles of Th1 and Th2 cytokines in a murine model of allergic dermatitis. J. Clin. Investig. 103:1103-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Su, N.-Y., C.-J. Yu, H.-D. Shen, F.-M. Pan, and L.-P. Chow. 1999. Pen c 1, a novel enzymic allergen protein from Penicillium citrinum: purification, characterization, cloning and expression. Eur. J. Biochem. 261:115-123. [DOI] [PubMed] [Google Scholar]
- 111.Summerbell, R. C. 1997. Epidemiology and ecology of onychomycosis. Dermatology 194(Suppl. 1):32-36. [DOI] [PubMed] [Google Scholar]
- 112.Szczeklik, A., and D. D. Stevenson. 2003. Aspirin-induced asthma: advances in pathogenesis, diagnosis, and management. J. Allergy Clin. Immunol. 111:913-921. [DOI] [PubMed] [Google Scholar]
- 113.Tagami, H., S. Watanabe, S. Ofuji, and K. Minami. 1977. Trichophytin contact sensitivity in patients with dermatophytosis. Arch. Dermatol. 113:1409-1414. [PubMed] [Google Scholar]
- 114.Wan, H., H. L. Winton, C. Soeller, E. R. Tovey, D. C. Gruenert, P. J. Thompson, G. A. Stewart, G. W. Taylor, D. R. Garrod, M. B. Cannell, and C. Robinson. 1999. Der p 1 facilitates transepithelial allergen delivery by disruption of tight junctions. J. Clin. Investig. 104:123-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ward, G. W., G. Karlsson, G. Rose, and T. A. E. Platts-Mills. 1989. Trichophyton asthma: sensitization of bronchi and upper airways to dermatophyte antigen. Lancet i:859-862. [DOI] [PubMed] [Google Scholar]
- 116.Ward, G. W., J. A. Woodfolk, M. L. Hayden, S. Jackson, and T. A. E. Platts-Mills. 1999. Treatment of late-onset asthma with fluconazole. J. Allergy Clin. Immunol. 104:541-546. [DOI] [PubMed] [Google Scholar]
- 117.Weary, P. E., and J. L. Guerrant. 1967. Chronic urticaria in association with dermatophytosis. Response to the administration of griseofulvin. Arch. Dermatol. 95:400-401. [PubMed] [Google Scholar]
- 118.Werfel, T., M. Hentschel, A. Knapp, and H. Renz. 1997. Dichotomy of blood- and skin-derived IL-4 producing allergen-specific T cells and restricted V repertoire in nickel-mediated contact dermatitis. J. Immunol. 158:2500-2505. [PubMed] [Google Scholar]
- 119.Wilson, B. B., B. Deuell, and T. A. Platts-Mills. 1993. Atopic dermatitis associated with dermatophyte infection and Trichophyton hypersensitivity. Cutis 51:191-192. [PubMed] [Google Scholar]
- 120.Wise, F., and M. B. Sulzberger. 1930. Urticaria and hay fever due to trichophytin (Epidermophyton interdigitale). JAMA 95:1504. [Google Scholar]
- 121.Wood, S. R., and C. N. D. Cruickshank. 1962. The relation between trichophytin sensitivity and fungal infection. Br. J. Dermatol. 74:329-335. [DOI] [PubMed] [Google Scholar]
- 122.Woodfolk, J. A., and T. A. E. Platts-Mills. 2001. Diversity of the human allergen-specific T cell repertoire associated with distinct skin test reactions: delayed-type hypersensitivity-associated major epitopes induce Th1- and Th2-dominated responses. J. Immunol. 167:5412-5419. [DOI] [PubMed] [Google Scholar]
- 123.Woodfolk, J. A., J. B. Slunt, B. Deuell, M. L. Hayden, and T. A. E. Platts-Mills. 1996. Definition of a Trichophyton protein associated with delayed hypersensitivity in humans: evidence for immediate (IgE and IgG4) and delayed type hypersensitivity to a single protein. J. Immunol. 156:1695-1701. [PubMed] [Google Scholar]
- 124.Woodfolk, J. A., S. J. Sung, D. C. Benjamin, J. K. Lee, and T. A. E. Platts-Mills. 2000. Distinct human T cell repertoires mediate immediate and delayed-type hypersensitivity to the Trichophyton antigen, Tri r 2. J. Immunol. 165:4379-4387. [DOI] [PubMed] [Google Scholar]
- 125.Woodfolk, J. A., L. M. Wheatley, R. V. Piyasena, D. C. Benjamin, and T. A. E. Platts-Mills. 1998. Trichophyton antigens associated with IgE antibodies and delayed type hypersensitivity. J. Biol. Chem. 273:29489-29496. [DOI] [PubMed] [Google Scholar]
- 126.Yeang, H. Y., S. A. Arif, F. Yusof, and E. Sunderasan. 2002. Allergenic proteins of natural rubber latex. Methods 27:32-45. [DOI] [PubMed] [Google Scholar]
- 127.Yu, M., and G. Tomasa. 1993. A double blind, prospective randomized trial of ketoconazole, a thromboxane synthetase inhibitor, in the prophylaxis of the adult respiratory distress syndrome. Crit. Care Med. 21:1335-1344. [DOI] [PubMed] [Google Scholar]
- 128.Zaias, N., A. Tosti, G. Rebell, R. Morelli, F. Bardazzi, H. Bieley, M. Zaiac, B. Glick, B. Paley, M. Allevato, and R. Baran. 1996. Autosomal dominant pattern of distal subungual onychomycosis caused by Trichophyton rubrum. J. Am. Acad. Dermatol. 34:302-304. [DOI] [PubMed] [Google Scholar]
- 129.Zollner, T. M., M. Podda, R. Kaufmann, T. A. Platts-Mills, and J. A. Woodfolk. 2002. Increased incidence of skin infections in atopy: evidence for an antigen-specific homing defect? Clin. Exp. Allergy 32:180-185. [DOI] [PubMed] [Google Scholar]