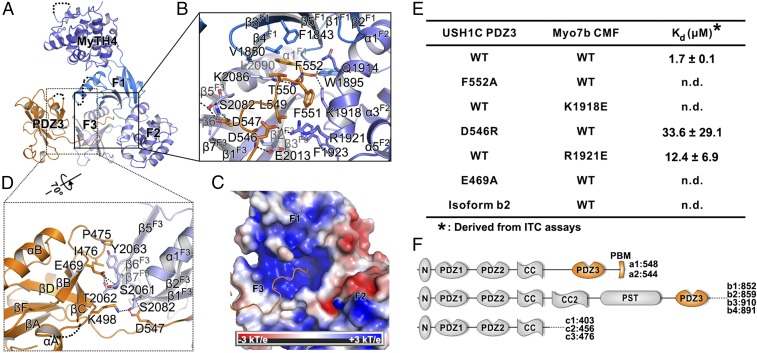
Fig. 2.
Detailed interaction between Myo7b CMF and USH1C PDZ3. (A) Ribbon representation of the Myo7b CMF/USH1C PDZ3 complex structure with the two major interfaces highlighted with the black boxes. (B) Detailed interaction between USH1C C-terminal tail and Myo7b CMF central pocket. Residues involved in binding are highlighted with stick models. Salt bridges and hydrogen bonds are indicated with dashed lines. (C) Surface electrostatic potential of the Myo7b CMF showing the positively charged central pocket. (D) Detailed interaction between USH1C PDZ3 βB/βC loop and Myo7b CMF F3 lobe. (E) Summary of dissociation constants showing that mutations of the critical residues in the interface either weakened or even abolished the binding. (F) Schematic diagrams showing domains organizations of different classes of USH1C spliced isoforms.