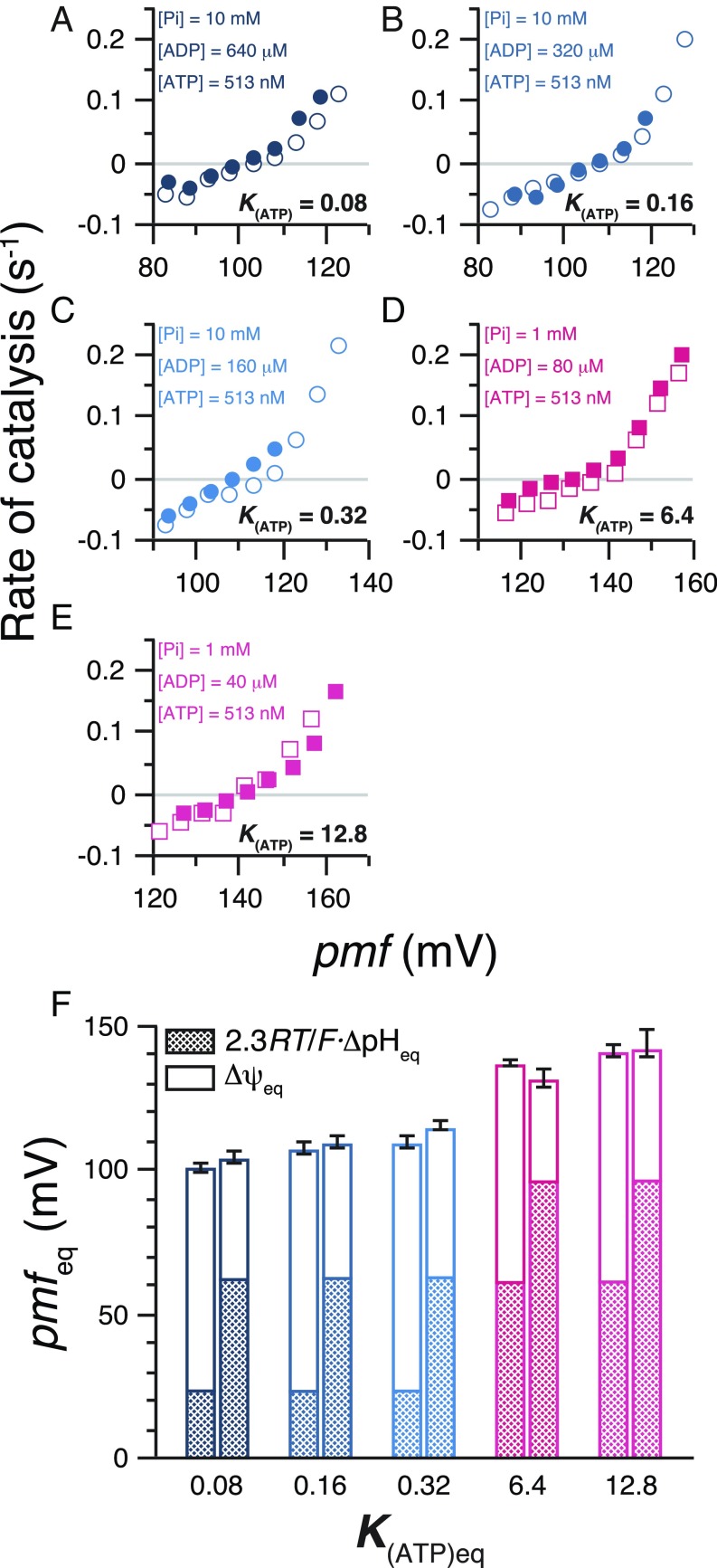
Fig. 4.
Equivalent contribution of ΔpH and Δψ to ATP synthesis/hydrolysis. (A−E) Kinetic equivalence of ΔpH and Δψ. A pair of rate-vs.-pmf plots under the same conditions except different combinations of ΔpH and Δψ that gives the same pmf. [ATP], [ADP], and [Pi] are indicated. ΔpH was set to 1.04 or 1.06 (open circles and open squares in A−E), 0.40 (filled circles in A−C), and 1.64 units (filled squares in D and E), and Δψ was changed by changing [K+]out. Error bars are omitted for clarity. (F) Thermodynamic equivalence of ΔpH and Δψ terms. Pairs of datasets with the same K(ATP)eq and pmfeq were selected, and contributions of Δψ and ΔpH in each dataset (bar) are shown with opened and meshed areas, respectively. Colors of the bars are same as those in A−E. Detailed conditions of the measurements are summarized in Tables S1 and S2.