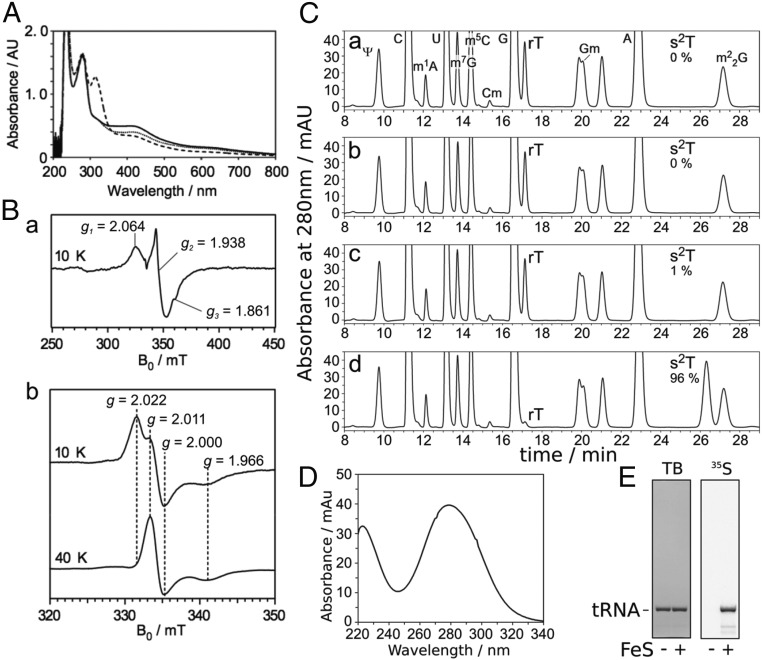
Fig. 2.
The iron-sulfur cluster on TtuA is required for s2T synthesis in vitro. (A) UV-VIS spectrum of TtuA. Reconstituted TtuA (solid line), TtuA treated with dithionite (dashed line), and TtuA treated with ferricyanide (dotted line) are shown. TtuA was diluted to 1.0 mg/mL in buffer A. The peak at around 310 nm in the reduced sample was derived from excess dithionite. (B) EPR spectrum of TtuA treated with dithionite at 10 K (a), and EPR spectra of TtuA treated with ferricyanide at 10 K (b, Upper) and 40 K (b, Lower). Principal g values are shown. (C) Nucleoside analysis of yeast tRNAPhe reacted in vitro. Apo-TtuA was treated with DTT (a), DTT and FeCl3 (b), DTT and Na2S (c), or DTT, FeCl3, and Na2S (d). Using these TtuA samples, tRNA (450 pmol) was reacted at 60 °C for 1 h with 75 pmol TtuA, TtuB, TtuC, TtuD, and SufS in the presence of 0.1 mM Cys and 2.5 mM ATP. The modified nucleosides of reacted RNA were analyzed by HPLC. The amounts of s2T formed are indicated; complete conversion of rT to s2T was set to 100%. (D) UV spectrum of s2T detected at 26.3 min in C, d. (E) Incorporation of 35S-sulfur to s2T from [35S]Cys. Yeast tRNAPhe (450 pmol) was reacted at 60 °C for 1 h with 75 pmol apo- or holo-TtuA, TtuB, TtuC, TtuD, and SufS in the presence of 0.1 mM [35S]Cys and 2.5 mM ATP. The reacted RNA was separated by 10% (wt/vol) denaturing PAGE and then stained with toluidine blue (TB; Left), after which 35S radioactivity was visualized (Right).