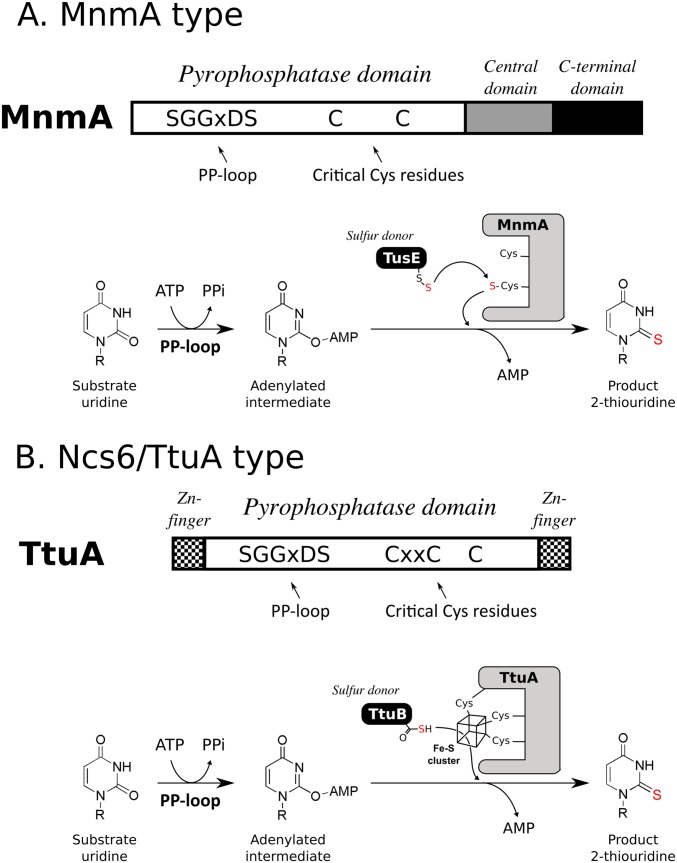
Fig. S1.
Schematic representation of the characteristics of MnmA-type (A) and Ncs6/TtyA-type (B) sulfurtransferases. Orientation of the PP-loop and critical Cys residues in the pyrophosphatase domain (Top), as well as the respective sulfur donor and reaction mechanism (Bottom) of MnmA and TtuA, are shown for comparison. The following characteristics of TtuA noted in this study are shown: TtuA has an [4Fe-4S] cluster; this cluster is essential for sulfur transfer activity; and the detailed role of the cluster remains unclear. The transferred sulfur atoms are colored in red.