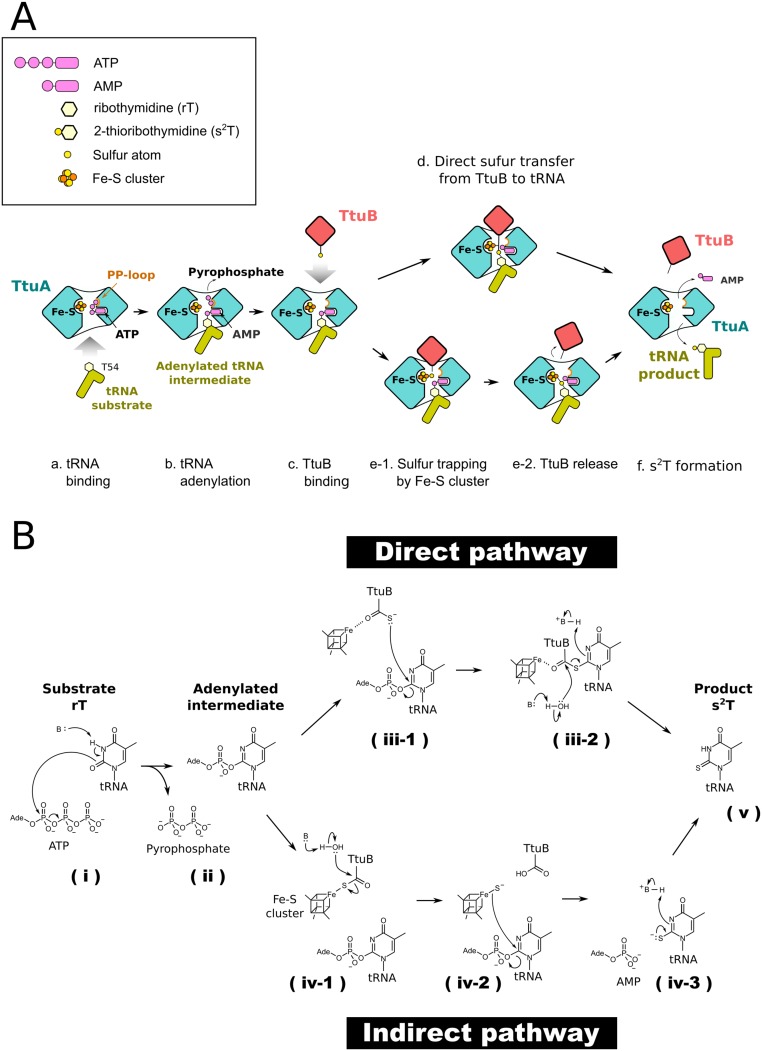
Fig. S7.
Proposed sulfur transfer mechanism by TtuA-TtuB using the iron-sulfur cluster. (A) Schematic representation of the proposed sulfur transfer mechanism. First, substrate tRNA binds to TtuA (a), which is followed by the transfer of the adenyl group of ATP to the substrate base of tRNA; an activated intermediate is then formed (b). Next, the sulfur-carrier protein TtuB inserts the thiocarboxylated C terminus into the active site for supplying the sulfur atom (c). Two possible mechanisms involving the Fe-S cluster have been proposed: the sulfur atom is transferred directly from TtuB to a uridine base, in which the Fe with a free coordination site is used for fixing the position of the C terminus of TtuB (d), or, alternatively, the sulfur atom, once released from TtuB, is captured by the Fe with a free coordination site of the [4Fe-4S] cluster (e-1) and is subsequently transferred to the activated uridine base (e-2). After completion of the thiolation reaction, the sulfur transfer complex is decomposed (f). (B) Deduced chemical reaction of the sulfur transfer. After the binding of substrate rT to TtuA, a basic residue would enhance the nucleophilicity of the O2 of rT by deprotonation of the N3, followed by a nucleophilic attack from O2 to alpha-phosphate of ATP (i), which generates the adenylated intermediate and the pyrophosphate (ii). Two possible mechanisms involving the Fe-S cluster have been proposed (Discussion and A). In the direct pathway, the Fe with a free coordination site is used for fixing the position of the C terminus of TtuB. The anionic S atom attacks position 2 of the rT, which causes the second substitution of the S for the AMP (iii-1). A water molecule deprotonated by a basic residue attacks the carbonyl carbon of TtuB C terminus and causes an elimination-addition reaction. The S atom is released and forms a double bond with the carbon of position 2. Subsequently, the nitrogen of position 3 is protonated by a basic residue (iii-2). In the indirect pathway, a water molecule deprotonated by a basic residue attacks the carbonyl carbon of TtuB C terminus that is coordinated by the cluster, causing an elimination-addition reaction. The released S atom remains on the Fe with a temporary free coordination site (iv-1). Subsequently, the S atom attacks position 2 of the rT, which causes the second substitution of the S for the AMP (iv-2). The S atom deprotonated by a basic residue forms double bond with the carbon of position 2, and the nitrogen of position 3 is protonated by a water molecule (iv-3). The thiolation reaction is completed and generates the product s2T (v).