Figure 11. Proposed mechanism of lesion formation in canine and human bestrophinopathies.
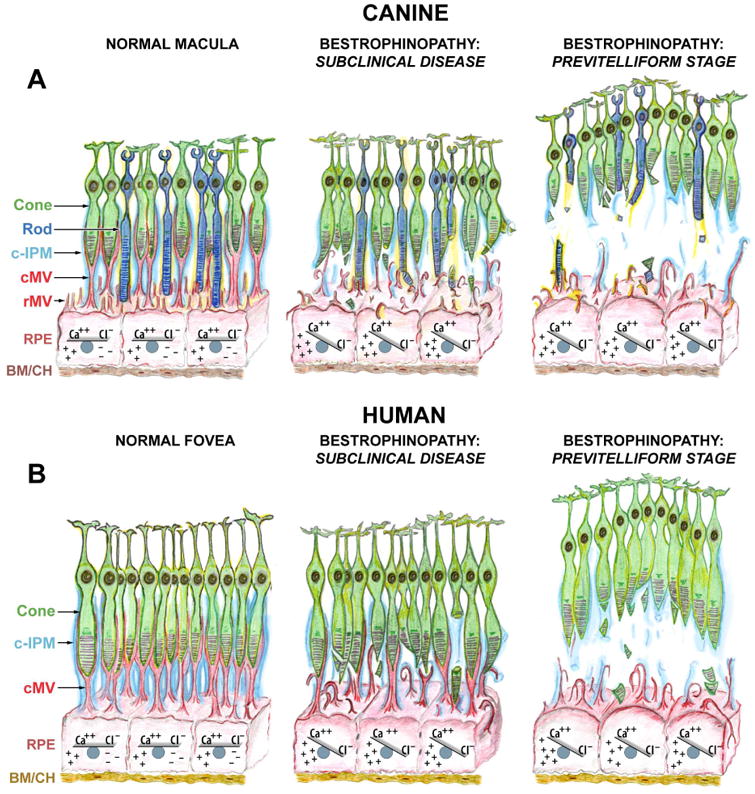
(A) Schematic representation of canine macula (a.k.a. area centralis) illustrating the normal RPE-photoreceptor apposition, sustained by intra- and extracellular ionic equilibria (Normal Macula, left panel). Ionic imbalance caused by mutation(s) in BEST1 gene initially affects RPE cell homeostasis, including expression of Ca++-dependent molecules (i. a., EZRIN). Impaired expression of EZRIN, the key determinant in the maturation of surface differentiations of RPE, leads to the underdeveloped RPE apical surface with fewer and rather immature microvilli that are not capable of investing and supporting the photoreceptor outer segments (Bestrophinopathy: Subclinical Disease, middle panel). Once the disease progresses, RPE cells gradually hypertrophy and lose their ability to mediate the ionic milieu of subretinal space. The cumulative effect of biochemical insult, accompanied by the structural abnormalities at the RPE apical surface weakens the adhesive forces of RPE-photoreceptor complex, which consequently results in separation of neuroretina from the RPE, and formation of serous lesion (Previtelliform Stage, right panel). (B) Schematic representation of human fovea. This rod-free structure is densely packed with cone photoreceptors tightly enveloped by RPE apical microvilli (cMV) and ensheathed in cone-specific interphotoreceptor matrix (c-IPM) (Normal Fovea, left panel). Due to the extremely high density of cones at this site, their outer segments in vivo will appear thinner in diameter, resembling rod photoreceptors; however, the cone outer segments are shorter than that of rods, and the RPE apical projections form long pedicles in order to reach them (cMV, red). The downstream effects of BEST1 mutation(s) manifest as both the ionic disequilibrium within RPE cells (and later within the adjacent subretinal space) and structural disintegration associated with RPE-derived cone ensheathment (Bestrophinopathy: Subclinical Disease, middle panel). As a consequence, the underdeveloped cone-associated RPE apical extensions are not able to physically support cone OS, which together with the reduced RPE functional capacity resulting directly from the decreased apical surface area, leads to the formation of macular-selective subretinal lesions at this highly metabolically active site (Bestrophinopathy: Previtelliform Stage, right panel). The compromised cone-associated insoluble IPM (c-IPM, cyan) may result from the chronic metabolic insult perturbing the delicate microenvironment of subretinal space and, secondarily, from the physical disassociation of the RPE-photoreceptor complex. The rate of disintegration of this extracellular matrix may constitute an important factor directly related to the expansion of vitelliform lesion and disease progression. Cone: cone photoreceptor cell; Rod: rod photoreceptor cell; c-IPM: cone-associated interphotoreceptor matrix; cMV: cone-associated microvilli; rMV: rod-associated microvilli; RPE: retinal pigment epithelium; BM/CH: Bruch's membrane/choroid.
