Figure 2.
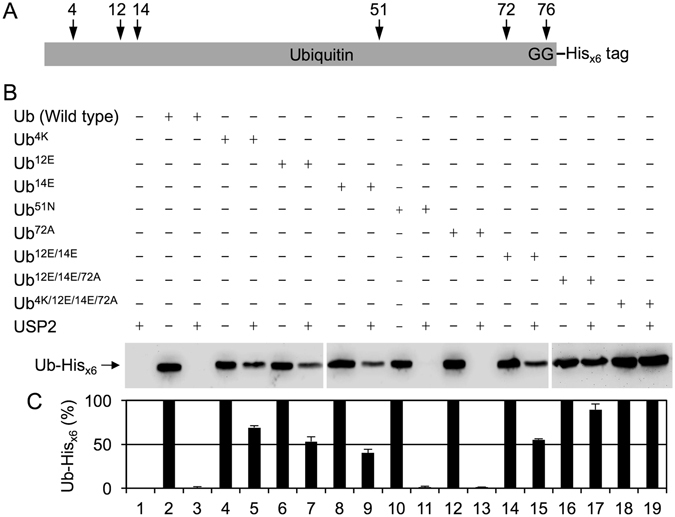
Residues at positions 4, 12, 14, and 72 of Ub are the molecular determinants for specific binding and catalysis by USP2. (A) A schematic diagram of the primary structure of the recombinant Ub used in the study, whose C-terminal di-glycine motif was fused with a Hisx6 tag (noted as Ub-Hisx6). USP2 cleaves on the normal peptide bond between the di-glycine motif (noted as GG) of Ub and the Hisx6 tag. The key residues of Ub interacting with USP2 were also labeled and marked with arrows. (B) Wild-type Ub-Hisx6 and indicated mutants (9.2 μM) were incubated with or without USP2 (4.75 μM) at 37 °C for 60 min. All reactions were terminated by adding 4X SDS-PAGE sample buffer and incubating at 100 °C for 10 min. Samples were separated on 16.6% SDS-PAGE and further analyzed using western blotting with the anti-Hisx6 tag antibody. Data are representative of three independent experiments. The full-length blots are presented in Supplementary Figure S1. (C) The signal intensity of three independent experiments was measured by a densitometer and processed by ImageJ. The results of the measurement were interpreted as a bar graph. Values are means ± S.D. from three independent experiments. The numbers noted at the bottom represent the lane numbers on the western blot.
