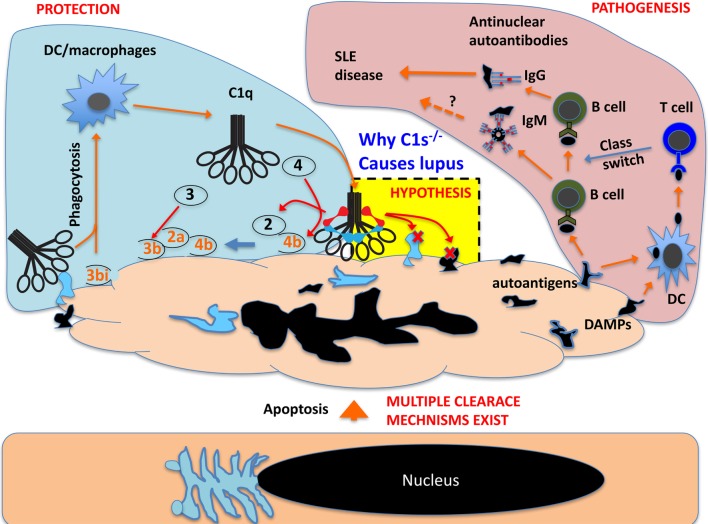
Figure 4.
Schematic proposal how C1 deficiency may cause systemic lupus erythematosus pathogenesis. In live cells, the nucleus and other intracellular structures are compartmentalized and excluded from complement recognition. When cells undergo apoptosis, the nucleus and other cellular structures disintegrate and, in late apoptotic cells, these fragments are recognized by C1q, which opsonize apoptotic cells for phagocytosis. This can also cause C1r/C1s activation and the activated C1s could cleave its classic substrate C4 and C2 and produce complement opsonins for phagocytosis. C1s may also cleave numerous exposed nuclear and other cellular proteins that are otherwise autoimmunogenic (autoantigens) and cause B cell production of autoantibodies. C1s may also cleave cellular proteins that are otherwise pro-inflammatory danger-associated molecular patterns (DAMPs) and activate DCs to cause B cell production of pathogenic IgG autoantibodies. C1s may not inactivate all autoantigens but effective inactivation of DAMPs can abrogate class switch of autoantibodies from IgM to pathogenic IgG.