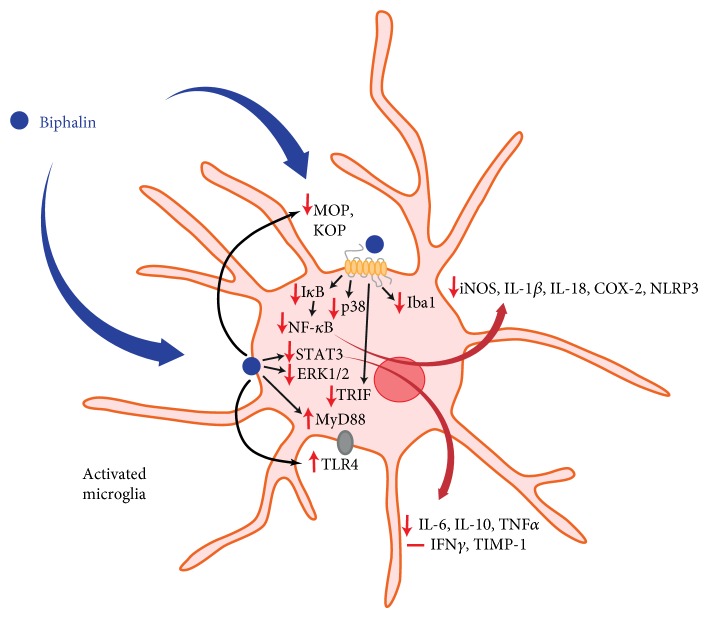
Scheme 1.
Hypothetical participation of biphalin in microglia-induced neuroinflammation. We suggest that the analgesic effects of biphalin during neuropathic pain are correlated with diminished microglia-induced neuroinflammation. Administration of biphalin reduces the activity of intracellular pathways in opioid receptor-dependent (NF-κB, p38) and opioid receptor-independent (STAT3, ERK1/2) manners in primary cultures of microglia. Biphalin treatment reduces activation of the NF-κB inhibitor, IκB, and enhances the STAT3 inhibitor, SOCS3. In response to the modulation of intracellular pathways by biphalin, the production of pronociceptive factors is diminished (iNOS, IL-1β, IL-18, COX-2, NLPR3, IL-6, and TNFα). Biphalin diminishes expression of microglial marker of cell activation (Iba1). Biphalin also modulates TLR4-related pathways, as well attenuates MOP receptor level to restore the homeostasis of the cell and reduce microglia activation. Our present data, as well as earlier reports [13, 17, 18], strongly suggest that biphalin can be considered as a promising therapeutic agent for the treatment of pain and other CNS pathologies correlated with neuroinflammation. Abbreviations: MOP, mu opioid receptor; DOP, delta opioid receptor; KOP, kappa opioid receptor; ERK1/2, extracellular signal-regulated kinase 1/2; STAT3, signal transducers and activators of transcription 3; SOCS3, suppressor of cytokine signaling 3; NF-κB, nuclear factor-κB; IκB, inhibitor of nuclear factor-κB; iNOS, inducible nitric oxide synthase; IL, interleukin; NLRP3, nucleotide-binding domain- (NOD-) like receptor protein 3; COX-2, cyclooxygenase 2; TNFα, tumor necrosis factor α; IFNγ, interferon γ; Iba1, ionized calcium-binding adaptor molecule 1; TLR4, Toll-like receptor 4; MyD88, myeloid differentiation primary response gene 88; and TRIF, TIR-domain-containing adapter-inducing interferon-β.