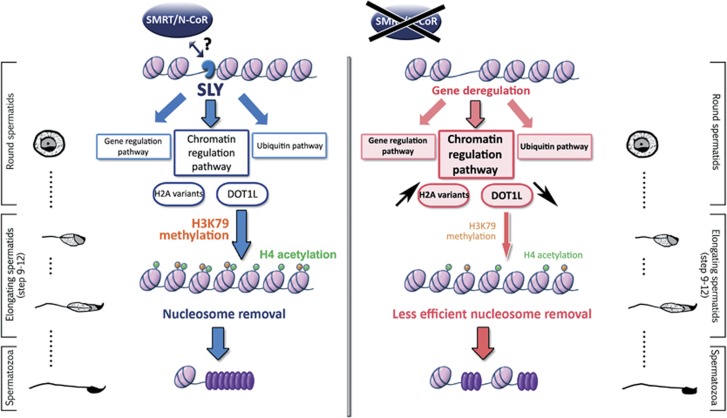
Figure 8.
Model presenting the mechanism by which SLY controls gene expression and chromatin remodeling during sperm differentiation. In WT round spermatids (left panel), SLY (in blue) interacts with the SMRT/N-CoR complex (which comprises TBL1XR1, TBL1X, NCOR1 and HDAC3) and is located at the start of genes involved in gene regulation, chromatin regulation and the ubiquitin pathway. In particular, SLY directly controls the expression of X-chromosome-encoded genes coding for H2.A variants (such as H2A.B3) and of the H3K79 methyltransferase DOT1L. In elongating spermatids, there is a wave of H3K79 dimethylation (orange circles) and of histone H4 acetylation (green circles); those modifications are expected to be a prerequisite to the efficient removal of nucleosomes (light pink oval) and replacement by protamines (purple oval), a process which is required to achieve optimal compaction of the spermatozoa nucleus. When SLY is knocked down (right panel), X-encoded H2.A variants are upregulated and more incorporated in the spermatid chromatin, while DOT1L is downregulated. DOT1L downregulation leads to a decrease in dimethylated H3K79 and acetylated histone H4 in elongating spermatids. Alterations in the spermatid chromatin structure affect the replacement of nucleosomes by protamines and lead to a higher proportion of nucleosomes and a decreased proportion of protamines. As a result, Sly-deficient spermatozoa are abnormally shaped, less compact and present a higher susceptibility to DNA damage than WT spermatozoa