Figure 1.
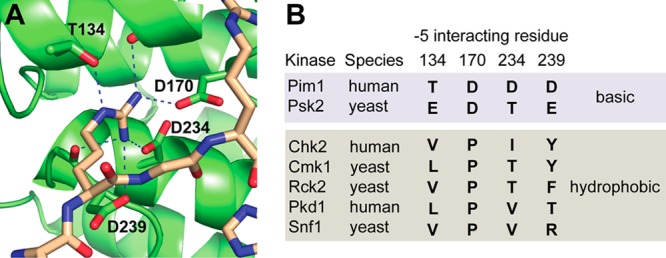
Residues in the −5 interaction pocket of Pim1 and related kinases. (A) The crystal structure of Pim1 in complex with a peptide substrate, showing the four residues (Thr134, Asp170, Asp234, and Asp239) that make direct contact with an Arg residue at the −5 position. (B) Residues found at the analogous positions in related kinases that select basic (top, shaded blue) or hydrophobic (bottom, shaded gray) residues at the −5 position on the basis of published peptide array analyses.14,23,30
