ABSTRACT
The Shigella species cause millions of cases of watery or bloody diarrhea each year, mostly in children in developing countries. While many aspects of Shigella colonic cell invasion are known, crucial gaps in knowledge regarding how the bacteria survive, transit, and regulate gene expression prior to infection remain. In this study, we define mechanisms of resistance to bile salts and build on previous research highlighting induced virulence in Shigella flexneri strain 2457T following exposure to bile salts. Typical growth patterns were observed within the physiological range of bile salts; however, growth was inhibited at higher concentrations. Interestingly, extended periods of exposure to bile salts led to biofilm formation, a conserved phenotype that we observed among members of the Enterobacteriaceae. Characterization of S. flexneri 2457T biofilms determined that both bile salts and glucose were required for formation, dispersion was dependent upon bile salts depletion, and recovered bacteria displayed induced adherence to HT-29 cells. RNA-sequencing analysis verified an important bile salt transcriptional profile in S. flexneri 2457T, including induced drug resistance and virulence gene expression. Finally, functional mutagenesis identified the importance of the AcrAB efflux pump and lipopolysaccharide O-antigen synthesis for bile salt resistance. Our data demonstrate that S. flexneri 2457T employs multiple mechanisms to survive exposure to bile salts, which may have important implications for multidrug resistance. Furthermore, our work confirms that bile salts are important physiological signals to activate S. flexneri 2457T virulence. This work provides insights into how exposure to bile likely regulates Shigella survival and virulence during host transit and subsequent colonic infection.
KEYWORDS: Shigella, bile salts, biofilm, resistance, EPS matrix, virulence genes, Escherichia coli, virulence
INTRODUCTION
The Shigella species are Gram-negative, facultative intracellular pathogens that cause illness by invading the colonic epithelium. Shigellosis results in significant morbidity and mortality around the globe each year, predominately in children under the age of 5 years in developing countries (1–3). There is currently no vaccine against Shigella, and antibiotic resistance is an increasing complication, particularly in areas with limited medical care (4). In order to invade epithelial cells, the bacteria utilize a type III secretion system (T3SS), the Ipa proteins required for invasion, and other effector proteins required to maintain infection encoded by a 200- to 220-kb virulence plasmid (3).
While many aspects of the Shigella invasion process, its intracellular survival, and the immune response have been thoroughly investigated, there is a significant gap in knowledge regarding how the bacterium successfully reaches the colonic epithelium to establish infection. During host gastrointestinal transit, the bacteria are exposed to numerous stimuli, particularly bile in the small intestine. Bile is an essential component of digestion and is composed of proteins, lipids, carbohydrates, vitamins, mineral salts, and other trace elements (5). In the liver, primary bile acids are synthesized by hepatocytes and are further metabolized by conjugation to glycine or taurine through N-acyl amidation. At physiological pH, conjugated bile acids are almost fully ionized and are termed bile salts (5, 6). Bile salts are maintained in the duodenum, jejunum, and proximal ileum to solubilize, digest, and absorb lipids and lipid-soluble vitamins (5, 6). In the distal ileum, bile salts are absorbed into the bloodstream, complexed to plasma proteins, and returned to the liver (6). The majority of bile (95%) is recycled back into the small intestine and does not enter the colon (7). In the small intestine, concentrations of bile salts range from 0.2% to 2% (wt/vol) depending on time of day, diet, and the individual (8). Bile is bactericidal (9, 10); however, Gram-negative bacteria, particularly enteric pathogens, are known to resist the bactericidal activity of bile and utilize this host component as a localization signal to regulate virulence gene expression and enhance infection (11).
Bile salts have been previously demonstrated to increase the ability of Shigella flexneri to adhere to and invade epithelial cells (12–15). Additionally, we have previously identified a mechanism of the bile salt-induced adherence phenotype by demonstrating that bile salts increase the expression of the ospE1 and ospE2 genes and subsequently promote the outer membrane localization of the proteins to function as adhesins (16). In order to further understand how S. flexneri 2457T survives host transit, we analyzed the ability of the bacteria to resist bile salts under various growth conditions. We identified that prolonged periods of exposure to bile salts led to biofilm formation in Shigella, a phenotype that we demonstrate is conserved among other enteric bacteria. Biofilm formation required the presence of glucose, whereas biofilm dispersion required removal of bile salts from the medium. Subsequent experiments demonstrated that S. flexneri 2457T released from the biofilm had an induced adherence phenotype relative to bacteria grown in traditional medium. RNA-sequencing analysis identified important changes in gene expression profiles during exposure to bile salts, including transcriptional alterations of key virulence genes and genes encoding products involved in resistance to bile salts. Finally, targeted mutagenesis experiments confirmed the importance of the predicted bile salt resistance genes. This work complements our previous analysis and provides us with a more complete understanding of how S. flexneri 2457T resists bile exposure and likely utilizes this host signal to induce virulence factor expression prior to entry into the colon. Insights gained from this work could enhance the development of novel therapeutics or vaccines.
RESULTS
Growth curve analysis of S. flexneri in the presence of bile salts.
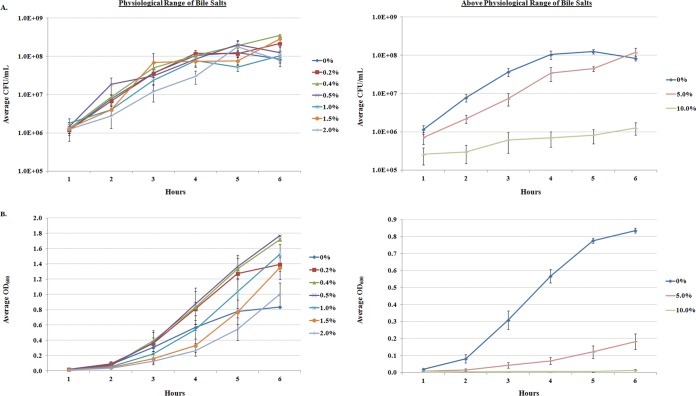
S. flexneri 2457T was grown in tryptic soy broth (TSB) or Luria-Bertani broth (LB) in the presence of various concentrations of bile salts (Fig. 1; see also Fig. S1 in the supplemental material). We examined growth at the physiological range of bile salts, 0.2% to 2% (wt/vol) (8), as well as at concentrations above the physiological range, i.e., at 5% and 10% (wt/vol). S. flexneri 2457T grew normally within the physiological range of bile salts as determined by CFU per milliliter, but growth was significantly slowed at 5% (wt/vol) bile salts and inhibited (bacteriostatic) at 10% (wt/vol) bile salts. The data indicate that S. flexneri 2457T can withstand the normal concentrations of bile salts found in the human gastrointestinal tract (8). For the TSB analysis, longer growth within the physiological range led to the formation of bacterial aggregates and white precipitation in the cultures (see below), which was not observed above the physiological range of bile salts. The aggregation and precipitation phenotype was reflected by sharp increases in the optical density readings relative to those taken in media without bile salts (Fig. 1B). These increases in optical density did not correlate with increases in bacterial growth (Fig. 1A). All subsequent analyses were performed in 0.4% (wt/vol) bile salts, since growth was determined to not be significantly altered at this concentration, the aggregation phenotype was statistically significant (Fig. 1B), and these conditions induced an adherence phenotype as previously described (16).
FIG 1.
Growth curve analysis of S. flexneri 2457T in bile salts. Bacterial growth was monitored in TSB medium or medium containing increasing concentrations of bile salts. (A) Average CFU/ml ± standard errors (SE) for three independent experiments are plotted. S. flexneri 2457T grew normally within the physiological range of 0.2% to 2.0% (wt/vol), but growth was slowed and inhibited at 5% and 10% (wt/vol), respectively. Statistical significance relative to the 0% control was detected at 5% and 10% (wt/vol) with P values of 0.001. (B) The corresponding average OD600 ± SE are plotted. In the physiological range of bile salts, sharp increases in optical density were detected. Statistical significance relative to the 0% control was detected at 0.4%, 5%, and 10% (all wt/vol) with P values ranging from <0.05 to 0.0001.
Prolonged exposure to bile salts induces biofilm formation.
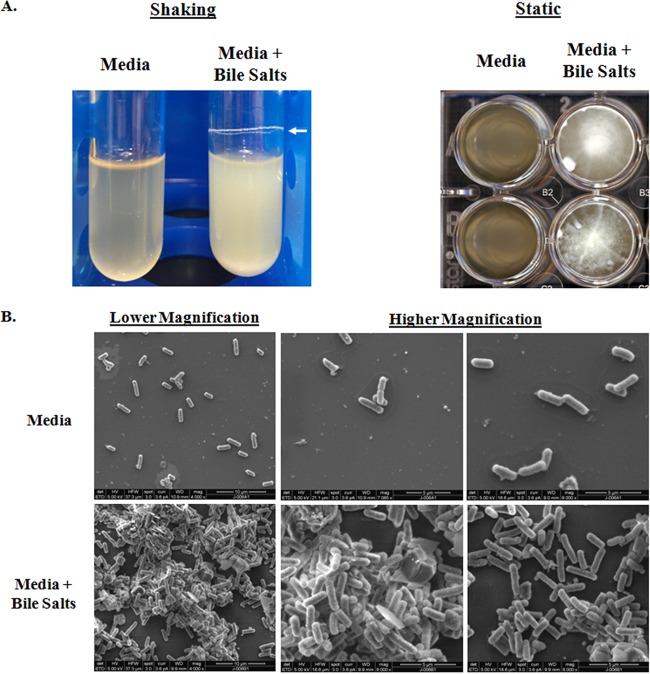
The longer periods of bile salt exposure in TSB (3 to 6 h under shaking conditions or overnight static growth) resulted in the formation of bacterial aggregates, clumping, and white precipitation in the culture (Fig. 2A). This phenotype was not detected in cultures exposed to bile salts in LB (data not shown). Scanning electron microscopy (SEM) analysis of static overnight cultures verified the observations (Fig. 2B). Bacteria cultured in TSB were dispersed on glass coverslips and were easily removed during the gentle washing step prior to fixing for SEM analysis. However, bacteria grown in TSB with 0.4% (wt/vol) bile salts adhered to the coverslips and bacterial aggregates were visualized during SEM analysis.
FIG 2.
Prolonged exposure to bile salts leads to clumping of the bacteria. (A) Bacteria subcultured for 4 h under shaking conditions at 225 rpm (left) or single colonies inoculated into each well of a 24-well plate and grown statically overnight (right) in medium with or without bile salts. The bacteria exposed to 0.4% (wt/vol) bile salts clumped and formed an adhesive ring (white arrow) around the culture tube or to the bottom of the wells. Images are representative of three independent experiments. (B) Scanning electron microscopy analysis of bile salt-induced clumping. Bacteria were grown on coverslips in medium alone (top) or in the presence of 0.4% (wt/vol) bile salts (bottom), fixed, and processed for SEM analysis. In medium alone, the bacteria were dispersed on the coverslips. Following exposure to bile salts, the bacteria clumped and adhered better to the coverslips. Images are representative of three independent experiments. Lower magnification images (×4,000; scale bars, 10 μm) are on the left, and higher magnification images (×7,000 to ×9,000; scale bars, 5 μm) are in the middle and on the right.
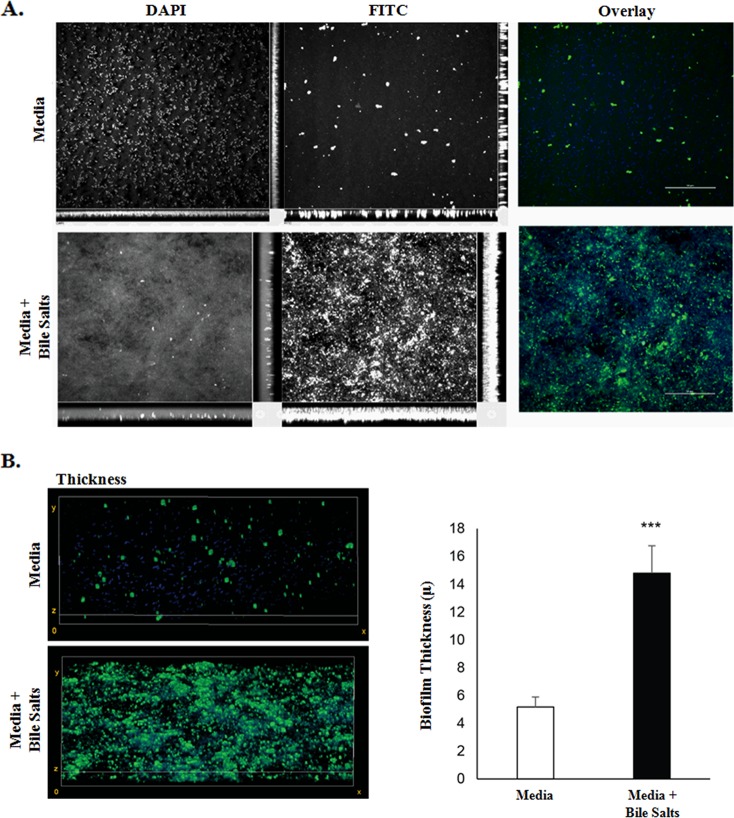
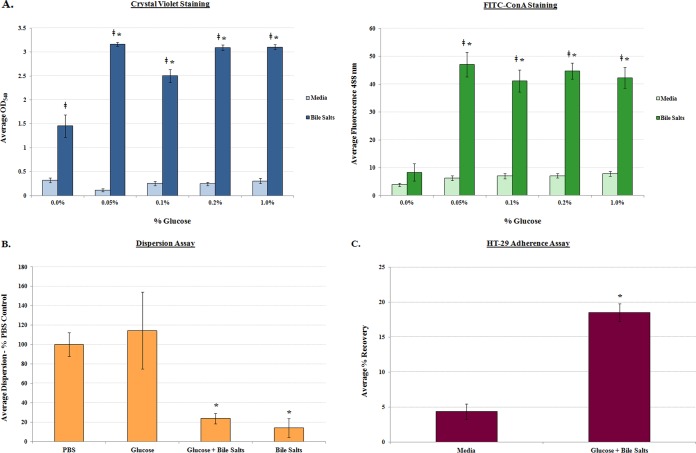
Given that the appearance of bacterial aggregates and the clumping phenotype resembled biofilm formation by other pathogens (17–22), additional assays were performed to verify and quantify the observations. First, crystal violet staining was performed based on previous observations of bile-induced biofilm formation in Vibrio cholerae (17). In the absence of bile salts, biofilm formation was minimal; however, there was a significant induction of biofilm formation in the presence of bile salts (Fig. 3). Second, since biofilm formation is characterized by extracellular polymeric substances (EPS) that consist mostly of polysaccharides whose production can be visualized through concanavalin A (ConA) staining (23, 24), we performed this analysis on S. flexneri 2457T overnight static cultures. In the absence of bile salts, EPS production and the overall presence of aggregates were minimal. However, in the presence of bile salts, EPS production, aggregate frequency, and thickness significantly increased (Fig. 4). We further demonstrated that EPS production was dependent on the presence of glucose (Fig. 5A), the removal of bile salts led to biofilm dispersion (Fig. 5B), and S. flexneri 2457T released from the biofilm had significantly induced adherence to HT-29 cells (Fig. 5C). Finally, we were interested in determining if the bile salt-induced biofilm formation was conserved among the enteric pathogens within the Enterobacteriaceae family. We therefore tested isolates of both commensal and pathogenic strains of Escherichia coli as well as Salmonella enterica serovars Typhi and Typhimurium. As shown in Table 1, most strains of E. coli and both S. Typhi and S. Typhimurium formed a biofilm in this assay in the presence of bile salts.
FIG 3.
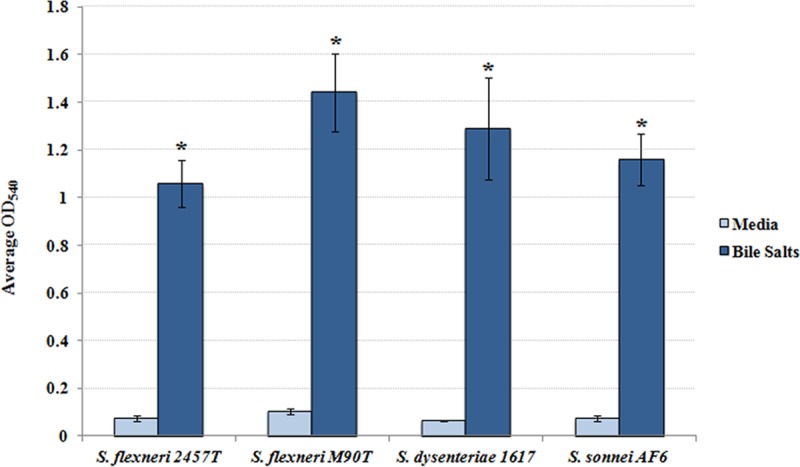
Quantification of biofilm formation. Single colonies of the indicated bacterial strains were inoculated into TSB ± 0.4% (wt/vol) bile salts, grown overnight at 37°C, and subsequently processed with crystal violet staining. In medium without bile salts, biofilm formation was minimal; however, following bile salt exposure, biofilm formation was significantly induced in all strains of Shigella tested (*, P < 0.0001). The average OD540 ± SE values from three independent experiments are presented. There were no significant differences between bacterial strains.
FIG 4.
Analysis of exopolysaccharide matrix production. (A) S. flexneri 2457T was grown on glass coverslips in TSB ± 0.4% (wt/vol) bile salts, fixed, and stained with DAPI (blue) and 25 μg/ml FITC-conjugated concanavalin A (green) to assess EPS production. Bacteria in medium alone were dispersed and had few areas staining positive for concanavalin A, while bacteria exposed to bile salts clumped and had more concanavalin A staining. The images are representative of three independent experiments. (B) To quantify the thickness of the biofilms, image stacks were taken every 0.25 μm of the biofilm, and quantification was determined from the full thickness of the stack. The image on the left represents 3D reconstructed stacks using ImageJ software. The average thickness (±SE) of each biofilm from three independent experiments is plotted on the right. There was a significant difference in biofilm thickness of S. flexneri 2457T following exposure to bile salts (***, P < 0.01).
FIG 5.
Analysis of biofilm formation, dispersion, and subsequent adherence to HT-29 cells. (A) Analysis of glucose requirement in biofilm formation. Single colonies of S. flexneri 2457T were inoculated into medium (LB) with or without 0.4% (wt/vol) bile salts at the indicated percentage (wt/vol) of glucose. Crystal violet (left) and FITC-ConA (right) staining was performed, and the average OD540 and fluorescence at 488 nm, respectively, ± SE are presented. Bile salts increased bacterial aggregation, and the addition of glucose further increased this phenotype. However, EPS production was dependent on the presence of glucose. For both the crystal violet and FITC-ConA staining, all differences between medium alone and the corresponding condition with bile salts are indicated with a double dagger (‡, P < 0.001). Differences in biofilm formation between each of the glucose plus bile salts concentrations relative to 0.0% glucose plus bile salts concentration are indicated with an asterisk (*, P < 0.01). (B) Analysis of bile salt removal in biofilm dispersion. S. flexneri 2457T biofilms were gently washed with PBS and resuspended in prewarmed PBS or PBS with the indicated treatments for 30 min at 37°C. The supernatants were subsequently removed and dilution plated, and CFU were determined. Dispersion was minimal in the presence of bile salts (*, P < 0.01), regardless of the presence of glucose. The average dispersion ± SE relative to the PBS control is plotted for three independent experiments in which technical triplicates were performed for each assay. For reference, the average recovery for the PBS treatment was 7.6 × 107 CFU while the average recovery for the PBS with bile salts treatment was 7.8 × 105 CFU. (C) Analysis of S. flexneri 2457T adherence following dispersion from the biofilm. Bacteria were grown overnight in media (LB) without or with a combination of glucose and bile salts to initiate biofilm formation. On the next day, bacteria were collected, washed with 1× PBS, and analyzed for adherence to HT-29 cells. Strain 2457T consistently had significantly increased adherence following biofilm dispersion (*, P < 0.0001) compared to bacteria recovered from medium without glucose and bile salts. The average percent recovery of adherent bacteria ± SE is plotted for three independent experiments, each of which had technical triplicates.
TABLE 1.
Biofilm analysis of additional bacterial strains
| Straina | Bile salts | Avg OD540b | SE | P valuec |
|---|---|---|---|---|
| E. coli F18 | − | 0.156 | ±0.038 | |
| + | 1.712 | ±0.108 | <0.0001 | |
| E. coli HS | − | 0.220 | ±0.018 | |
| + | 0.249 | ±0.019 | Not significant | |
| E. coli MC4100 | − | 0.201 | ±0.039 | |
| + | 1.189 | ±0.116 | <0.0001 | |
| EHEC 86-24 | − | 0.015 | ±0.021 | |
| + | 1.216 | ±0.070 | <0.0001 | |
| EHEC 933 | − | 0.014 | ±0.013 | |
| + | 1.399 | ±0.140 | <0.0001 | |
| STEC 98NK2 | − | 0.088 | ±0.035 | |
| + | 0.747 | ±0.097 | <0.0001 | |
| EPEC E2348/69 | − | 0.005 | ±0.002 | |
| + | 0.323 | ±0.058 | 0.0001 | |
| EAEC O42 | − | 0.228 | ±0.017 | |
| + | 0.257 | ±0.024 | Not significant | |
| EAEC Δaaf | − | 0.012 | ±0.004 | |
| + | 0.282 | ±0.039 | 0.0001 | |
| S. Typhi Ty2 | − | 0.053 | ±0.007 | |
| + | 0.505 | ±0.165 | 0.04 | |
| S. Typhimurium SL1344 | − | 0.009 | ±0.003 | |
| + | 0.210 | ±0.036 | 0.0001 |
EHEC, enterohemorrhagic E. coli; STEC, Shiga toxin-producing E. coli; EPEC, enteropathogenic E. coli; EAEC, enteroaggregative E. coli.
The average OD540 for the crystal violet quantification of biofilm formation was measured for three independent experiments.
P values based on the Student t test between media with or without bile salts for each of the strains tested.
RNA-seq analysis identifies differentially expressed genes following exposure to bile salts.
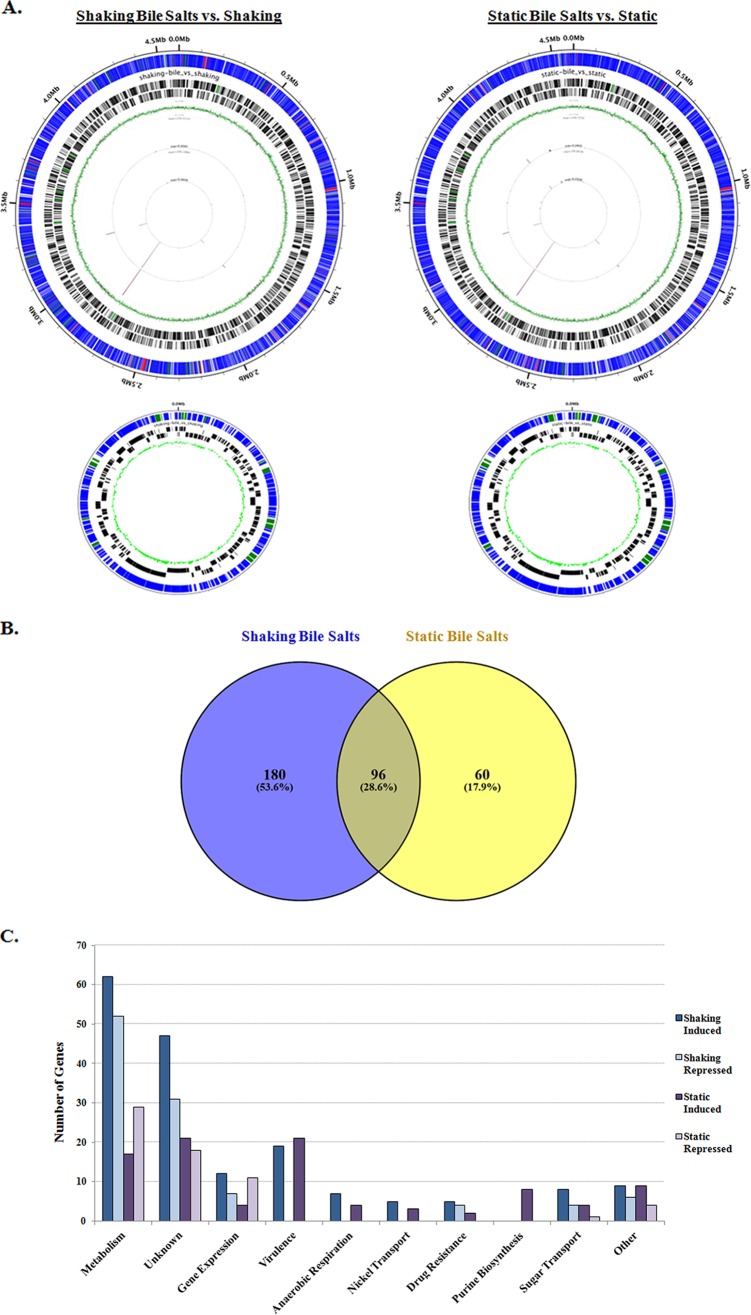
We performed high-throughput RNA sequencing (RNA-seq) analysis of S. flexneri 2457T grown in the presence and absence of bile salts under both shaking and static conditions to identify patterns of gene expression, with differential expression defined as induced or repressed genes with a 2-fold or greater change in expression and a P value cutoff of ≤0.05. During shaking growth, there were a total of 276 differentially expressed genes in the cultures grown in the presence of bile salts compared to those in media without bile salts, of which 172 genes were induced and 104 genes were repressed (Fig. 6; see also Tables S2 and S3 in the supplemental material). During static growth, there were a total of 156 differentially expressed genes in the presence of bile salts compared to media without bile salts, of which 93 genes were induced and 63 genes were repressed. There were 96 differentially expressed genes that occurred under both shaking and static conditions (Fig. 6B). Overall, induced genes in the presence of bile salts most notably comprised genes involved in central metabolism (pur, glp, mgl, nik genes), gene expression (tdcA, yhjC, and rhaS), sugar transporters (fruAB, ptsG, and treB), drug resistance (acrAB, fsr, and yegB), and virulence (several osp and ipaH genes, including ospE1 and ospE2) (Fig. 6C; Tables S2 and S3). The analysis confirmed many of the microarray findings previously identified by our group (16) and allowed us to extend the gene expression studies to include bile salts under static growth conditions. Furthermore, the increased sensitivity and specificity of RNA-seq allowed us to detect additional transcriptional changes in the presence of bile salts, especially on the virulence plasmid (Tables S2 and S3).
FIG 6.
RNA-sequencing analysis of S. flexneri 2457T grown in the presence and absence of 0.4% (wt/vol) bile salts. (A) Results of the RNA-seq analysis visualized with the Circleator program (71), in which blue represents unchanged genes, green represents induced genes (fold change ≥ 2, P < 0.05), and red represents repressed genes (fold change ≤ −2, P < 0.05) in the presence of bile salts for both the chromosome (top) and virulence plasmid (bottom). Barcodes represent forward and reverse genes, while the green circle represents the percent GC skew throughout the genome. (B) Venn diagram (72) of differentially expressed genes identified in the RNA-seq analysis. The numbers of induced and repressed genes for the “shaking + bile salts versus shaking” condition compared to the “static + bile salts versus static” condition are depicted. For both comparisons, there were 96 genes that were induced or repressed in the presence of bile salts. For the “shaking + bile salts versus shaking” analysis, there were an additional 180 genes induced or repressed, for a total of 276 differentially expressed genes in bile salts. For the “static + bile salts versus static” analysis, there were an additional 60 genes that were induced or repressed, for a total of 156 differentially expressed genes in bile salts. (C) Functional categories for differentially expressed genes in the presence of bile salts are plotted with the number of genes induced or repressed under the bile salts condition for both the shaking and static growth conditions. Please refer to Tables S2 and S3 in the supplemental material for details.
In order to verify the RNA-seq results, we selected several genes to analyze via quantitative reverse-transcription (qRT)-PCR analysis using three biologically independent samples of RNA to compare the expression levels in the presence of bile salts with those in media without bile salts under shaking growth conditions (Fig. 7). We chose two highly induced genes, S2558 and ospE1-ospE2, two induced genes, acrB and yhcN, a repressed gene, dsdA, and an independently regulated control gene, yacG. In all, qRT-PCR analysis confirmed the induction of S2558, ospE1-ospE2, acrB, and yhcN in the presence of bile salts, the repression of dsdA, and the unchanged transcription of yacG.
FIG 7.
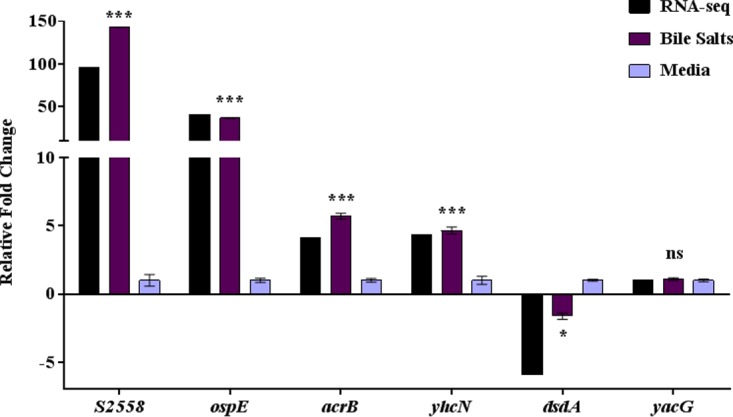
Quantitative reverse transcription-PCR analysis verifies the RNA-sequencing analysis. qRT-PCR was performed on three independent RNA samples isolated from S. flexneri 2457T grown with 0.4% (wt/vol) bile salts (Bile Salts) or without (Media). The relative fold changes ± SE from the ΔCT values are plotted for the 6 genes. The corresponding changes in gene expression from the RNA-seq analysis are provided as a reference in black. ospE indicates both ospE1 and ospE2. Significance symbols for the qRT-PCR data of expression of genes in bile salts compared to the expression of genes in media without bile salts: ***, P < 0.0001; *, P < 0.05; n.s., not significant.
Identification of mutants unable to grow in bile salts.
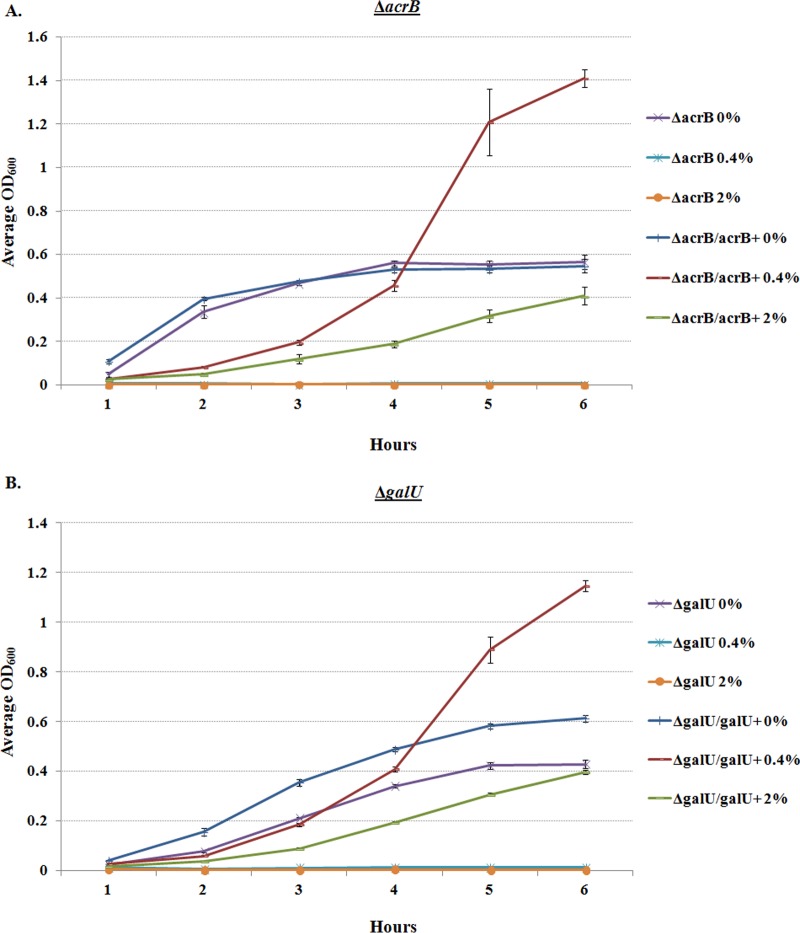
In conjunction with our RNA-seq results and the literature characterizing bile resistance in other enteric pathogens (11), we analyzed a mutant in the AcrAB multidrug efflux pump (ΔacrB) and a ΔgalU mutant that lacks the O-antigen of the lipopolysaccharide (LPS) (25, 26). Both mutants had growth patterns similar to those of the wild type in unsupplemented medium; however, the mutants were unable to grow in either 0.4% or 2.0% (wt/vol) bile salts. Complementation restored the bile salt resistance phenotype (Fig. 8). The data confirm the importance of the AcrAB efflux pump and complete LPS for S. flexneri 2457T resistance to bile salts.
FIG 8.
Growth analysis of mutants in the presence of bile salts. The ΔacrB (A) and ΔgalU (B) mutants were analyzed for growth in TSB ± 0.4% and 2% (wt/vol) bile salts. The graphs depict the growth curve analysis of three independent experiments, with the average OD600 ± SE plotted. The mutants were unable to grow in either 0.4% or 2% bile salts (P < 0.001), and complementation restored growth in both 0.4% and 2% bile salts (P < 0.001).
DISCUSSION
Bile is a multifunctional, essential component of digestion. In humans, it is secreted into the duodenum to solubilize and digest fats and lipids as food transits the small intestine (6). Bile also solubilizes lipid-based microbial membranes to function as an endogenous antimicrobial compound (9). Many enteric pathogens have developed multiple strategies to resist the bactericidal effects of bile while also utilizing this host signal to regulate virulence factor expression (11). For the first time, this work identifies bile salt resistance strategies used by Shigella that we hypothesize are required to successfully transit the small intestine prior to infection in the colon. Moreover, this work provides further evidence that Shigella utilizes bile as a signal to adapt to an intestinal environment and facilitate virulence gene expression prior to infection in the colon.
Resistance to bile is accomplished through several approaches in enteric pathogens (9, 11). One resistance strategy repeatedly observed in Salmonella, Vibrio, Campylobacter, and E. coli is the removal of bile compounds through the use of several types of efflux pumps (11, 27–34). Efflux pumps are utilized by bacteria to expel antibiotics and other harmful substances from the bacterial cytosol. The AcrAB efflux pump is a member of the resistance nodulation division (RND) family of efflux pumps, which represents one of the five families associated with multidrug resistance (MDR) (35). The increased expression of the AcrAB efflux pump in the presence of bile salts not only would enable Shigella to resist the bactericidal effects of bile but could also potentially “prime” the bacteria for an antibiotic resistance phenotype. In fact, a recent study evaluating the bile salt effects on enterohemorrhagic E. coli (EHEC) found that increased expression of acrAB transcripts, as well as increased expression of lipopolysaccharide genes, conferred resistance to the antibiotic polymyxin (30). Furthermore, overexpression of AcrB has been linked to MDR resistance in S. Typhimurium (36, 37). Therefore, the ability to combat MDR in enteric pathogens is potentially further complicated by the simple fact that these pathogens are exposed to bile during the infection process. This concept is an important consideration for future antimicrobial strategies to combat MDR and treat infections resulting from enteric pathogens.
The presence of LPS, specifically the O-antigen, is important for bile resistance in enteric pathogens, as various rough mutants or mutations in galU, which catalyzes the formation of UDP-glucose and is required for O-antigen synthesis, lead to bile salt sensitivity in Salmonella, E. coli, and Vibrio (9, 32, 38, 39). Likewise, in our study, loss of galU and therefore the complete O-antigen (26) abolished Shigella growth capability in the presence of bile salts. Analysis in Salmonella has demonstrated that both remodeling of lipid A and elongation of the O-chains of LPS are important for bile resistance (40–42). LPS length has also been demonstrated to be important for Shigella virulence. The S. flexneri ΔgalU mutant exhibited a defect in adherence to and invasion of polarized intestinal epithelial cells (25) and failed to efficiently spread from cell to cell due to a lack of proper IcsA unipolar localization (26). Future analysis will determine the Shigella LPS modifications that occur in the presence of bile and examine the subsequent effects on virulence.
Prolonged exposure of Shigella to bile salts resulted in increased biofilm formation, an important resistance mechanism for many bacterial pathogens. Biofilm formation is characterized by a surface-adherent, EPS matrix-protected prosurvival state and is a common approach for bacterial pathogens to resist antimicrobial exposures such as antibiotics, UV radiation, and pH stress (24). We hypothesize that bile salt-induced biofilm formation is an additional bile resistance strategy employed by Shigella. We are currently in the process of identifying and performing functional mutagenesis studies in genes required for EPS production to assess their effects on biofilm formation and bile salt resistance. Similar biofilm phenotypes have been described for Vibrio, Campylobacter, and Listeria as well as for Salmonella gallbladder colonization (11, 17, 43–46). Our work extends this observation to Shigella and additional enteric bacteria such as the pathogenic variants of E. coli and, as a result, demonstrates that bile salt-induced biofilm formation is conserved among members of the Enterobacteriaceae family. In keeping with the accepted paradigm that minimal numbers of Shigella are required for establishing an infection (3), we hypothesize that biofilm formation also promotes bacterial cell aggregation to ensure successful transit of the small intestine and entry into the colon for infection. As noted in our analysis here, and in other pathogens such as Vibrio (47, 48), virulence factor expression is also regulated during exposure to bile salts. Given the dual control of resistance to bile salts and virulence factor expression in the presence of bile salts, we propose that the enteric bile salt-induced biofilm is a transient phenotype compared to traditional biofilms produced by pathogens like Pseudomonas (24). The transient nature would allow a more rapid dispersion as the bacteria reach the epithelial cell surfaces in the small intestine or enter the colon, where bile concentrations are reduced relative to those of the lumen of the small intestine (6). In fact, to highlight this transient nature, our analysis demonstrated that there was significant biofilm dispersion following 30 min of removal of bile salts from the media. Interestingly, biofilm formation has been shown to require purine biosynthesis regulation of the bacterial second messenger cyclic-di-GMP (49), efflux pumps (50), and galU for EPS matrix production (38), further connecting our findings of the bile salt-induced gene expression profile and the bile salt ΔacrB and ΔgalU mutant phenotypes to biofilm formation. Future analysis will determine the effects of these mutations on biofilm formation and virulence.
We demonstrated that robust biofilm formation did not occur in LB medium and required the presence of glucose as well as bile salts. The requirement for glucose in biofilm formation, particularly for EPS production, has been documented for many pathogens, including Vibrio (51, 52), Listeria (53), Pseudomonas (54), Acinetobacter (55), and Staphylococcus (56). To further highlight the importance of glucose in biofilm formation, the RNA-seq analysis demonstrated that the ptsG gene, encoding the glucose-specific phosphoenolpyruvate phosphotransfer system (PTS) IIBC components, was induced in S. flexneri 2457T in the presence of bile salts as seen with Vibrio biofilms (51, 52). As food is digested in the small intestine, simple sugars such as glucose are available until these sugars are absorbed by host cells in the terminal ileum (57). Therefore, Shigella is exposed to both bile salts and glucose as it transits the majority of the small intestine to enable biofilm formation. Given that bile has additional components, including a few bile salts not analyzed here (6), future analysis of biofilm formation with the individual components of bile or bile extract in the presence and absence of glucose will confirm our findings.
Finally, this work provides further insights that bile acts as a host localization signal to prepare Shigella for entry into the colon. In conjunction with previous work demonstrating that bile salts induce Shigella adherence and invasion to epithelial cells (12–16), S. flexneri 2457T recovered from the biofilm had an increased adherence phenotype. This result is confirmed by the RNA-seq analysis in which there was significant induction of ospE1 and ospE2 genes that encode the bile salt-induced adhesins (16). Furthermore, induction of additional osp and ipaH genes in the presence of bile salts was detected, which we hypothesize would prepare the bacteria for an intracellular lifestyle, as many of these genes are important for maintaining infection in epithelial cells (3, 58). Interestingly, no virulence genes were repressed in our analysis; however, it is worth noting that genes encoding the Mxi-Spa T3SS and the Ipa invasion proteins were not induced in the presence of bile salts, using the conservative thresholds outlined. The mxi-spa and ipa operons are effectively induced at 37°C through VirF activation of the central transcriptional regulator virB, with VirB in turn regulating mxi-spa and ipa transcription (3). We hypothesize that additional induction of invasion genes in the small intestine by bile would be detrimental to Shigella by promoting a premature invasion phenotype and possibly preventing the ability of the bacteria to survive in the presence of bile. Bile salts trigger the recruitment of the invasins to the tip of the T3SS needle complex, which would be sufficient for Shigella to prepare for invasion (12, 14, 15). In addition to virulence gene inductions, we hypothesize that exposure to bile salts permits Shigella to prepare for the anaerobic environment encountered in the colon. The nikABCDE operon, which is important for nickel transport and has been shown to be required for survival under anaerobic conditions (59), was induced in bile salts. Also, the yegB gene was induced 2.8-fold in the presence of bile salts. This gene is a homolog to the efflux pump component mdtE, which has been shown to protect E. coli from toxic by-product accumulation during anaerobic respiration (60). In all, the induction of genes that support both virulence and exposure to an anaerobic environment further suggests that Shigella utilizes bile as a signal to prepare for infection in the low-oxygen environment of the colon.
In conclusion, this study identifies potential mechanisms by which Shigella resists the bactericidal effects of bile and provides further insights into how bile enhances a virulent phenotype in Shigella. We propose a model by which Shigella induces bile salt resistance and related biofilm formation phenotypes during transit of the small intestinal lumen while at the same time inducing virulence factor expression (Fig. 9). Based on our data, we propose that the reduction of available bile in the terminal ileum promotes Shigella biofilm dispersion, enabling subsequent adherence and invasion in the colon. Furthermore, this work highlights the importance of incorporating in vivo exposures or aspects of human physiology in studying host-microbe interactions. One of the well-known challenges in studying Shigella is the limited animal models available (61). Since Shigella is a human-adapted pathogen and bile composition varies from species to species with notable differences in routine laboratory animals such as rats, rabbits, and nonhuman primates (62), significant alterations in Shigella gene expression profiles following exposure to bile salts underscore the importance of adaptation to host physiology during pathogenesis analysis. Incorporation of preexposure of bile salts into in vitro and in vivo infection models for Shigella and other enteric pathogens may finally improve our approaches to developing cost-effective, long-lasting therapeutics and vaccines.
FIG 9.
Model of Shigella infection following bile exposure. As Shigella transits the small intestine, exposure to bile induces transcriptional changes, bile resistance mechanisms, and biofilm formation. In the terminal ileum, where the majority of bile is absorbed, Shigella disperses the biofilm and subsequently enters the colon. Transcriptional changes already induced in response to bile, such as increased anaerobic respiration and the induction of the osp and ipaH virulence genes, enable the bacteria to efficiently adapt to the colonic environment, attach and invade epithelial cells, and establish infection. The bacteria escape macrophages by inducing cell death, invade the basolateral pole of the colonic epithelium, and regulate interleukin-8 (IL-8) secretion to control polymorphonuclear (PMN) cell migration (3, 73–75).
MATERIALS AND METHODS
Bacterial strains and growth conditions used in the study.
Table 2 lists the bacterial strains and plasmids used in this study. Bacteria were routinely cultured at 37°C in either Luria-Bertani broth (LB) or tryptic soy broth (TSB, which contains an additional 2.5 g/liter glucose relative to LB), with aeration or in petri dishes to represent static growth conditions. Plating for CFU was performed using tryptic soy broth plates with 1.5% agar and 0.025% Congo red (CR; Sigma). Bile salts (Sigma B8756, consisting of an approximate 1:1 mixture of cholate and deoxycholate) were used at a concentration of 0.4%, wt/vol, unless indicated otherwise. For the dose-dependent glucose analysis, the indicated amounts of glucose were added to LB. All media were filter sterilized with a 0.22-μm filter following the addition of bile salts and/or glucose. Chloramphenicol was used at 5 μg/ml, ampicillin at 100 μg/ml, and tetracycline at 10 μg/ml when indicated.
TABLE 2.
Bacterial strains and plasmids used in this study
| Name | Description | Source or reference |
|---|---|---|
| Strains | ||
| Shigella | ||
| 2457T | S. flexneri serotype 2a | 76 |
| M90T | S. flexneri serotype 5 | 77 |
| 1617 | S. dysenteriae | 78 |
| AF6 | S. sonnei (recent clinical isolate) | AFRIMSa |
| BS766 | 2457T transformed with pKM208 | 70 |
| ΔacrB | 2457T/acrB::cat, chloramphenicol resistance | This study |
| ΔacrB (pAcrB) | ΔacrB transformed with pAcrB | This study |
| BS109 | 2457T/galU::Tn10, tetracycline resistance | 26 |
| BS109 (pGalU) | BS109 transformed with pGalU | This study |
| E. coli | ||
| DH5α | Laboratory E. coli | 79 |
| F18 | Mouse commensal E. coli | 80 |
| HS | Human commensal E. coli | 81 |
| MC4100 | E. coli K-12 derivative | 82 |
| 86-24 | Enterohemorrhagic E. coli O157:H7 (Stx2) | 83 |
| 933 | Enterohemorrhagic E. coli O157:H7 (Stx1, Stx2) | 83 |
| STEC 98NK2 | Shiga toxin-producing E. coli O113:H21 strain | 84 |
| EPEC E2348/69 | Enteropathogenic E. coli archetype O127:H6 strain | 81 |
| EAEC O42 | Enteroaggregative E. coli archetype O42 | 85 |
| Δaaf strain | O42/aafA::TnphoA | 86 |
| Salmonella | ||
| Typhi | S. enterica serovar Typhi strain Ty2 | 87 |
| Typhimurium | S. enterica serovar Typhimurium strain SL1344 | 88 |
| Plasmids | ||
| pKD3 | oriR6K, bla, cat | 89 |
| pKM208 | Temperature-sensitive red-, gam-, lacI-expressing plasmid driven by PTac promoter, bla | 89 |
| pUC19 | Cloning vector, bla, high copy no. | 90 |
| pGEMT | PCR cloning vector, bla, high copy no. | Promega |
| pAcrB | pUC19::acrB, wild-type gene cloned into pUC19 | This study |
| pGalU | pGEMT::galU, wild-type gene cloned into pGEMT | This study |
Department of Enteric Diseases, Armed Forces Research Institute of Medical Sciences (AFRIMS), Bangkok, Thailand.
Growth curve analysis.
Bile salts were dissolved in sterile medium (LB or TSB) at the specified concentration and sterilized with a 0.22-μm filter. Overnight cultures of S. flexneri 2457T were diluted 1:50 into medium with or without the various concentrations of bile salts. Triplicate cultures were grown in a shaking incubator at 225 rpm at 37°C in a 96-well plate. The optical density at 600 nm (OD600) was measured every hour over a period of 6 h using a Spectramax plate reader with samples blanked against medium alone. Aliquots of the cultures were removed for dilution plating from each well after gently mixing at each time point to determine the CFU present in each well. For all assays, statistical significance was determined by a two-way analysis of variance (ANOVA) and a P value of ≤0.05 was considered significant.
Static growth analysis and biofilm assay.
Single colonies of each bacterial strain were inoculated into TSB ± 0.4% (wt/vol) bile salts in a single well of a 96-well plate. Plates were incubated at 37°C overnight without shaking. On the following day, wells were gently washed twice with 1× phosphate-buffered saline (PBS) and stained with 0.5% crystal violet for 5 min. Afterwards, the wells were gently washed five times with sterile distilled water (dH2O) and then set to air dry. Biofilm formation was quantified by adding 95% ethanol to the wells to solubilize the crystal violet stain. After 30 min of incubation at room temperature, absorbance at 540 nm (OD540) was measured with the plate reader (63). Samples were blanked to wells that were incubated with medium without bile salts and underwent the same staining procedure. For the dose-dependent glucose analysis, the indicated percentage of glucose was added to LB medium with or without 0.4% (wt/vol) bile salts, and the assay was performed as described above. Absorbance readings at OD600 were taken to ensure that there were no significant differences in growth prior to the washing steps. Statistical significance was determined by Student's t test (for comparisons of presence and absence of bile salts for each strain) or an ANOVA, and a P value of ≤0.05 was considered significant.
For biofilm dispersion analysis, S. flexneri 2457T biofilms were seeded as described above. On the following day, wells were gently washed twice with 1× PBS, and biofilms were resuspended in prewarmed PBS, PBS with 2% glucose, PBS with 2% glucose and 0.4% bile salts, or PBS with 0.4% bile salts and incubated at 37°C for 30 min. Afterwards, supernatants were collected, dilution plated onto CR plates, and incubated overnight to determine the CFU. Experiments were performed in biological triplicates, with technical triplicates for each experiment. Statistical significance was determined using an ANOVA, and a P value of ≤0.05 was considered significant.
Scanning electron microscopy.
In order to analyze the appearance of the outer surface of the bacteria in the presence of bile salts, SEM was performed. Single colonies were grown on coverslips in TSB ± 0.4% (wt/vol) bile salts and incubated at 37°C overnight. The coverslips were gently washed, placed in 3% glutaraldehyde fixative (in PBS), and sent for SEM processing by the Core Imaging Facility, University of Maryland School of Dentistry. Samples were imaged with a FEI Quanta 200 scanning electron microscope.
Analysis of exopolysaccharide matrix formation and confocal microscopy.
S. flexneri 2457T biofilms (in medium with or without 0.4% [wt/vol] bile salts) grown on glass coverslips were fixed with 3% paraformaldehyde and 0.25% gluteraldehyde in 1× PBS and subsequently stained with 25 μg/ml fluorescein isothiocyanate-conjugated concanavalin A (FITC-ConA; Sigma) for 15 min at room temperature. Stained and control cells were washed in 1× PBS, mounted using prolong antifade with 4′,6-diamidino-2-phenylindole (DAPI; Molecular Probes), and imaged using a Leica confocal microscope. To quantify the thickness of the biofilms, image stacks were taken every 0.25 μm of the biofilm, and quantification was determined from the full thickness of the stack. Three-dimensional (3D) reconstructed images were generated using ImageJ software (https://imagej.nih.gov). The average thicknesses from three independent experiments ± the standard errors (SE) were plotted to quantify differences in thickness. Statistical significance was determined by Student's t test, and a P value of ≤0.05 was considered significant. For quantification analysis, the biofilm assay was performed in black 96-well immunoplates and performed as described above. After the wash steps following overnight incubation, wells were treated with fixative (3% paraformaldehyde and 0.25% glutaraldehyde in 1× PBS) for 15 min. The wells were subsequently washed and stained with FITC-ConA for 15 min at room temperature. Each well was subsequently washed in 1× PBS, and the fluorescence at 488 nm was measured with a BioTek fluorescence plate reader. This analysis was performed in three independent experiments, and statistical significance was determined using a two-way ANOVA, with a P value of ≤0.05 considered significant.
HT-29 adherence assay.
The adherence assay was performed as previously described for T84 cells (16) with the following modifications. HT-29 cells (ATCC HTB-38) were seeded in Dulbecco's modified Eagle medium (DMEM) to establish a semiconfluent monolayer of approximately 75%. For bacterial cultures, single colonies of S. flexneri 2457T were inoculated into medium (LB) or medium with 2% glucose and 0.4% (wt/vol) bile salts in tissue culture plates and grown statically at 37°C. Following overnight growth, the bacteria were collected, washed with 1× PBS, standardized to an OD600 of 0.35, resuspended in DMEM, and applied to the HT-29 cell monolayers. Cells were incubated at 37°C with 5% CO2 for 3 h. Afterwards, the monolayers were washed five times with 1× PBS and lysed with 1% Triton X-100. Serial dilutions were made to determine the number of cell-associated bacteria. The average percent recovery was calculated for three independent experiments according to the following formula: (recovered bacterial titer/infecting titer) × 100%. Statistical significance was determined by Student's t test, and a P value of ≤0.05 was considered significant.
RNA isolation and RNA-seq analysis.
RNA was isolated from S. flexneri 2457T grown in TSB ± 0.4% (wt/vol) bile salts under shaking or static conditions. Isolation was performed on two independent, biological replicates for each growth condition (a total of 8 samples). All bacteria were grown to an OD600 of 0.7, approximately 2.5 h without bile salts and 3.0 h with bile salts for both shaking and static conditions. Bacterial RNA was isolated using the RNeasy kit (Qiagen) according to the manufacturer's protocol. DNase treatment was performed using Invitrogen's Turbo DNase, and concentrations of total RNA were determined using a NanoDrop ND-1000 spectrophotometer. To confirm RNA integrity, RNA was resolved on a Tris-borate-EDTA (TBE) agarose gel to ensure lack of degradation. Next, cDNA was synthesized from total RNA using the RevertAid cDNA first-strand synthesis kit (Thermo Scientific) according to the manufacturer's protocol followed by PCR amplification with 2× Taq reaction mix (Denville) of the housekeeping gene rpoA (see Table S1 in the supplemental material). All RNA was confirmed to be free of DNA contamination by performing separate cDNA synthesis reactions without reverse transcriptase, followed by PCR amplification of rpoA. RNA samples were sent to Genewiz for RNA-seq, with RNA integrity determined prior to sample processing through bioanalyzer analysis (Agilent 2100) by the company. All RNA integrity numbers (RIN) ranged from 9.7 to 10.0, confirming that the RNA was intact and not degraded (64). rRNA was depleted prior to RNA library preparation and multiplexing. Sequencing was performed on the Illumina HiSeq2500 platform, in a 1 × 50 bp single-read configuration.
The number of sequenced reads ranged from 17,044,246 to 20,194,826 with mean Phred quality (Q) scores ranging from 38.28 to 38.36, representing a 99.99% base call accuracy rate (65, 66). The RNA-seq analysis was performed as previously described (67). Briefly, the publicly available S. flexneri 2457T genome and S. flexneri 2a strain 301 virulence plasmid annotations were used in the data analysis. The transcript reads were aligned to the predicted coding regions, and the differential expression of each gene under the different conditions was determined using DESeq v. 1.5.24 (68). The reads aligned to each gene were normalized and then averaged for each of the two biological replicates, and the fold change and the log2 of the fold change (LFC) were calculated for each comparison. The expression data were then filtered for further analysis, and genes that had a fold change of ≥2 and ≤−2, a minimum normalized read count of 10, and a P value and false discovery rate (FDR) of ≤0.05 were determined to be transcriptionally altered using DESeq v. 1.5.24 and R-2.15.2.
qRT-PCR analysis.
To verify the RNA-seq results, qRT-PCR analysis was performed on biologically independent RNA samples isolated from the static or shaking cultures in the presence or absence of 0.4% (wt/vol) as described above. The cDNA was synthesized from total RNA and confirmed to be free of DNA contamination as described above. The various PCRs were performed with the primer sets listed in Table S1 in the supplemental material. Analysis by qRT-PCR was performed in a two-step reaction in which cDNA was synthesized using the RevertAid first-strand cDNA synthesis kit (Thermo), and quantitative PCR followed using the SYBR green detection method with a CFX Connect Real-Time PCR detection system (Bio-Rad). Data were collected and analyzed using the CFX Manager Software (Bio-Rad). All data were normalized to levels of rpoA and analyzed using the comparative cycle threshold (ΔCT) method (69). The expression levels of the target genes under the various conditions were compared using the relative quantification method (69). Real-time data are expressed as the relative changes in expression levels between cultures in medium with bile salts and those in medium without bile salts. Statistical significance was determined by Student's t test, and a P value of ≤0.05 was considered significant.
Genetic mutant construction.
The ΔacrB mutant was constructed using the λ red linear recombination method as previously described (70). PCR was used to amplify a chloramphenicol resistance cassette gene (cat) from pKD3 (Table 1) with 5′ and 3′ overhangs identical to the 5′ and 3′ regions of acrB. Chloramphenicol-resistant recombinants were identified and selected on chloramphenicol plates and subsequently screened via PCR using confirmation primers (Table S1) that annealed to unique regions up- and downstream of each gene to detect the size difference due to the insertion of the chloramphenicol cassette. For acrB complementation analysis, the acrB gene was amplified using high-fidelity Taq polymerase as previously described (70), digested with HindIII and BamHI, and ligated into digested pUC19. For galU complementation analysis, the promoter and galU gene were amplified and ligated into pGEMT as previously described (16). Ligation reactions were transformed into E. coli DH5α and selected on LB with 100 μg/ml ampicillin. Following sequencing analysis of the inserts, the plasmids were transformed into the appropriate S. flexneri 2457T mutants for growth curve analysis of bile salt sensitivity.
Supplementary Material
ACKNOWLEDGMENTS
We thank Anthony Maurelli, Bryan Hurley, Alessio Fasano, Marcia Goldberg, Brett Swierczewski, and Bobby Cherayil for the strains used in this study.
This work was supported by the National Institute of Allergy and Infectious Diseases Grants K22AI104755 (C.S.F.), U19AI110820 (D.A.R.), and 5T32AI095190-04 (J.R.S.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.01067-16.
REFERENCES
- 1.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque AS, Zaidi AK, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acacio S, Biswas K, O'Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne RM, Levine MM. 2013. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 2.Livio S, Strockbine NA, Panchalingam S, Tennant SM, Barry EM, Marohn ME, Antonio M, Hossain A, Mandomando I, Ochieng JB, Oundo JO, Qureshi S, Ramamurthy T, Tamboura B, Adegbola RA, Hossain MJ, Saha D, Sen S, Faruque AS, Alonso PL, Breiman RF, Zaidi AK, Sur D, Sow SO, Berkeley LY, O'Reilly CE, Mintz ED, Biswas K, Cohen D, Farag TH, Nasrin D, Wu Y, Blackwelder WC, Kotloff KL, Nataro JP, Levine MM. 2014. Shigella isolates from the global enteric multicenter study inform vaccine development. Clin Infect Dis 59:933–941. doi: 10.1093/cid/ciu468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schroeder GN, Hilbi H. 2008. Molecular pathogenesis of Shigella spp.: controlling host cell signaling, invasion, and death by type III secretion. Clin Microbiol Rev 21:134–156. doi: 10.1128/CMR.00032-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhattacharya D, Bhattacharya H, Sayi DS, Bharadwaj AP, Singhania M, Sugunan AP, Roy S. 2015. Changing patterns and widening of antibiotic resistance in Shigella spp. over a decade (2000-2011), Andaman Islands, India. Epidemiol Infect 143:470–477. doi: 10.1017/S0950268814000958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reshetnyak VI. 2013. Physiological and molecular biochemical mechanisms of bile formation. World J Gastroenterol 19:7341–7360. doi: 10.3748/wjg.v19.i42.7341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ridlon JM, Kang DJ, Hylemon PB. 2006. Bile salt biotransformations by human intestinal bacteria. J Lipid Res 47:241–259. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]
- 7.Hamer HM, De Preter V, Windey K, Verbeke K. 2012. Functional analysis of colonic bacterial metabolism: relevant to health? Am J Physiol Gastrointest Liver Physiol 302:G1–G9. doi: 10.1152/ajpgi.00048.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kristoffersen SM, Ravnum S, Tourasse NJ, Okstad OA, Kolsto AB, Davies W. 2007. Low concentrations of bile salts induce stress responses and reduce motility in Bacillus cereus ATCC 14579 [corrected]. J Bacteriol 189:5302–5313. doi: 10.1128/JB.00239-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Begley M, Gahan CG, Hill C. 2005. The interaction between bacteria and bile. FEMS Microbiol Rev 29:625–651. doi: 10.1016/j.femsre.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Merritt ME, Donaldson JR. 2009. Effect of bile salts on the DNA and membrane integrity of enteric bacteria. J Med Microbiol 58:1533–1541. doi: 10.1099/jmm.0.014092-0. [DOI] [PubMed] [Google Scholar]
- 11.Sistrunk JR, Nickerson KP, Chanin RB, Rasko DA, Faherty CS. 2016. Survival of the fittest: how bacterial pathogens utilize bile to enhance infection. Clin Microbiol Rev 29:819–836. doi: 10.1128/CMR.00031-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pope LM, Reed KE, Payne SM. 1995. Increased protein secretion and adherence to HeLa cells by Shigella spp. following growth in the presence of bile salts. Infect Immun 63:3642–3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barta ML, Guragain M, Adam P, Dickenson NE, Patil M, Geisbrecht BV, Picking WL, Picking WD. 2012. Identification of the bile salt binding site on IpaD from Shigella flexneri and the influence of ligand binding on IpaD structure. Proteins 80:935–945. doi: 10.1002/prot.23251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olive AJ, Kenjale R, Espina M, Moore DS, Picking WL, Picking WD. 2007. Bile salts stimulate recruitment of IpaB to the Shigella flexneri surface, where it colocalizes with IpaD at the tip of the type III secretion needle. Infect Immun 75:2626–2629. doi: 10.1128/IAI.01599-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stensrud KF, Adam PR, La Mar CD, Olive AJ, Lushington GH, Sudharsan R, Shelton NL, Givens RS, Picking WL, Picking WD. 2008. Deoxycholate interacts with IpaD of Shigella flexneri in inducing the recruitment of IpaB to the type III secretion apparatus needle tip. J Biol Chem 283:18646–18654. doi: 10.1074/jbc.M802799200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faherty CS, Redman JC, Rasko DA, Barry EM, Nataro JP. 2012. Shigella flexneri effectors OspE1 and OspE2 mediate induced adherence to the colonic epithelium following bile salts exposure. Mol Microbiol 85:107–121. doi: 10.1111/j.1365-2958.2012.08092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hung DT, Zhu J, Sturtevant D, Mekalanos JJ. 2006. Bile acids stimulate biofilm formation in Vibrio cholerae. Mol Microbiol 59:193–201. doi: 10.1111/j.1365-2958.2005.04846.x. [DOI] [PubMed] [Google Scholar]
- 18.Friedman L, Kolter R. 2004. Genes involved in matrix formation in Pseudomonas aeruginosa PA14 biofilms. Mol Microbiol 51:675–690. doi: 10.1046/j.1365-2958.2003.03877.x. [DOI] [PubMed] [Google Scholar]
- 19.Brown HL, Reuter M, Salt LJ, Cross KL, Betts RP, van Vliet AH. 2014. Chicken juice enhances surface attachment and biofilm formation of Campylobacter jejuni. Appl Environ Microbiol 80:7053–7060. doi: 10.1128/AEM.02614-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fenton M, Keary R, McAuliffe O, Ross RP, O'Mahony J, Coffey A. 2013. Bacteriophage-derived peptidase CHAP(K) eliminates and prevents staphylococcal biofilms. Int J Microbiol 2013:625341. doi: 10.1155/2013/625341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niu C, Robbins CM, Pittman KJ, Osborn jL, Stubblefield BA, Simmons RB, Gilbert ES. 2013. LuxS influences Escherichia coli biofilm formation through autoinducer-2-dependent and autoinducer-2-independent modalities. FEMS Microbiol Ecol 83:778–791. doi: 10.1111/1574-6941.12034. [DOI] [PubMed] [Google Scholar]
- 22.Srinandan CS, Elango M, Gnanadhas DP, Chakravortty D. 2015. Infiltration of matrix-non-producers weakens the Salmonella biofilm and impairs its antimicrobial tolerance and pathogenicity. Front Microbiol 6:1468. doi: 10.3389/fmicb.2015.01468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strathmann M, Wingender J, Flemming HC. 2002. Application of fluorescently labelled lectins for the visualization and biochemical characterization of polysaccharides in biofilms of Pseudomonas aeruginosa. J Microbiol Methods 50:237–248. doi: 10.1016/S0167-7012(02)00032-5. [DOI] [PubMed] [Google Scholar]
- 24.Gupta P, Sarkar S, Das B, Bhattacharjee S, Tribedi P. 2016. Biofilm, pathogenesis and prevention—a journey to break the wall: a review. Arch Microbiol 198:1–15. doi: 10.1007/s00203-015-1148-6. [DOI] [PubMed] [Google Scholar]
- 25.Kohler H, Rodrigues SP, McCormick BA. 2002. Shigella flexneri interactions with the basolateral membrane domain of polarized model intestinal epithelium: role of lipopolysaccharide in cell invasion and in activation of the mitogen-activated protein kinase ERK. Infect Immun 70:1150–1158. doi: 10.1128/IAI.70.3.1150-1158.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sandlin RC, Lampel KA, Keasler SP, Goldberg MB, Stolzer AL, Maurelli AT. 1995. Avirulence of rough mutants of Shigella flexneri: requirement of O antigen for correct unipolar localization of IcsA in the bacterial outer membrane. Infect Immun 63:229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bina JE, Mekalanos JJ. 2001. Vibrio cholerae tolC is required for bile resistance and colonization. Infect Immun 69:4681–4685. doi: 10.1128/IAI.69.7.4681-4685.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chatterjee A, Chaudhuri S, Saha G, Gupta S, Chowdhury R. 2004. Effect of bile on the cell surface permeability barrier and efflux system of Vibrio cholerae. J Bacteriol 186:6809–6814. doi: 10.1128/JB.186.20.6809-6814.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buckley AM, Webber MA, Cooles S, Randall LP, La Ragione RM, Woodward MJ, Piddock LJ. 2006. The AcrAB-TolC efflux system of Salmonella enterica serovar Typhimurium plays a role in pathogenesis. Cell Microbiol 8:847–856. doi: 10.1111/j.1462-5822.2005.00671.x. [DOI] [PubMed] [Google Scholar]
- 30.Kus JV, Gebremedhin A, Dang V, Tran SL, Serbanescu A, Barnett Foster D. 2011. Bile salts induce resistance to polymyxin in enterohemorrhagic Escherichia coli O157:H7. J Bacteriol 193:4509–4515. doi: 10.1128/JB.00200-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin J, Sahin O, Michel LO, Zhang Q. 2003. Critical role of multidrug efflux pump CmeABC in bile resistance and in vivo colonization of Campylobacter jejuni. Infect Immun 71:4250–4259. doi: 10.1128/IAI.71.8.4250-4259.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thanassi DG, Cheng LW, Nikaido H. 1997. Active efflux of bile salts by Escherichia coli. J Bacteriol 179:2512–2518. doi: 10.1128/jb.179.8.2512-2518.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenberg EY, Bertenthal D, Nilles ML, Bertrand KP, Nikaido H. 2003. Bile salts and fatty acids induce the expression of Escherichia coli AcrAB multidrug efflux pump through their interaction with Rob regulatory protein. Mol Microbiol 48:1609–1619. doi: 10.1046/j.1365-2958.2003.03531.x. [DOI] [PubMed] [Google Scholar]
- 34.Baucheron S, Nishino K, Monchaux I, Canepa S, Maurel MC, Coste F, Roussel A, Cloeckaert A, Giraud E. 2014. Bile-mediated activation of the acrAB and tolC multidrug efflux genes occurs mainly through transcriptional derepression of ramA in Salmonella enterica serovar Typhimurium. J Antimicrob Chemother 69:2400–2406. doi: 10.1093/jac/dku140. [DOI] [PubMed] [Google Scholar]
- 35.Piddock LJ. 2006. Multidrug-resistance efflux pumps—not just for resistance. Nat Rev Microbiol 4:629–636. doi: 10.1038/nrmicro1464. [DOI] [PubMed] [Google Scholar]
- 36.Piddock LJ, White DG, Gensberg K, Pumbwe L, Griggs DJ. 2000. Evidence for an efflux pump mediating multiple antibiotic resistance in Salmonella enterica serovar Typhimurium. Antimicrob Agents Chemother 44:3118–3121. doi: 10.1128/AAC.44.11.3118-3121.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blair JM, Richmond GE, Piddock LJ. 2014. Multidrug efflux pumps in Gram-negative bacteria and their role in antibiotic resistance. Future Microbiol 9:1165–1177. doi: 10.2217/fmb.14.66. [DOI] [PubMed] [Google Scholar]
- 38.Nesper J, Lauriano CM, Klose KE, Kapfhammer D, Kraiss A, Reidl J. 2001. Characterization of Vibrio cholerae O1 El tor galU and galE mutants: influence on lipopolysaccharide structure, colonization, and biofilm formation. Infect Immun 69:435–445. doi: 10.1128/IAI.69.1.435-445.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lacroix FJ, Avoyne C, Pinault C, Popoff MY, Pardon P. 1995. Salmonella typhimurium TnphoA mutants with increased sensitivity to biological and chemical detergents. Res Microbiol 146:659–670. doi: 10.1016/0923-2508(96)81063-1. [DOI] [PubMed] [Google Scholar]
- 40.Crawford RW, Keestra AM, Winter SE, Xavier MN, Tsolis RM, Tolstikov V, Baumler AJ. 2012. Very long O-antigen chains enhance fitness during Salmonella-induced colitis by increasing bile resistance. PLoS Pathog 8:e1002918. doi: 10.1371/journal.ppat.1002918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dalebroux ZD, Miller SI. 2014. Salmonellae PhoPQ regulation of the outer membrane to resist innate immunity. Curr Opin Microbiol 17:106–113. doi: 10.1016/j.mib.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alvarez-Ordonez A, Begley M, Prieto M, Messens W, Lopez M, Bernardo A, Hill C. 2011. Salmonella spp. survival strategies within the host gastrointestinal tract. Microbiology 157:3268–3281. doi: 10.1099/mic.0.050351-0. [DOI] [PubMed] [Google Scholar]
- 43.Svensson SL, Pryjma M, Gaynor EC. 2014. Flagella-mediated adhesion and extracellular DNA release contribute to biofilm formation and stress tolerance of Campylobacter jejuni. PLoS One 9:e106063. doi: 10.1371/journal.pone.0106063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gonzalez-Escobedo G, Gunn JS. 2013. Gallbladder epithelium as a niche for chronic Salmonella carriage. Infect Immun 81:2920–2930. doi: 10.1128/IAI.00258-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Begley M, Kerr C, Hill C. 2009. Exposure to bile influences biofilm formation by Listeria monocytogenes. Gut Pathog 1:11. doi: 10.1186/1757-4749-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prouty AM, Schwesinger WH, Gunn JS. 2002. Biofilm formation and interaction with the surfaces of gallstones by Salmonella spp. Infect Immun 70:2640–2649. doi: 10.1128/IAI.70.5.2640-2649.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gupta S, Chowdhury R. 1997. Bile affects production of virulence factors and motility of Vibrio cholerae. Infect Immun 65:1131–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schuhmacher DA, Klose KE. 1999. Environmental signals modulate ToxT-dependent virulence factor expression in Vibrio cholerae. J Bacteriol 181:1508–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim JK, Kwon JY, Kim SK, Han SH, Won YJ, Lee JH, Kim CH, Fukatsu T, Lee BL. 2014. Purine biosynthesis, biofilm formation, and persistence of an insect-microbe gut symbiosis. Appl Environ Microbiol 80:4374–4382. doi: 10.1128/AEM.00739-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kvist M, Hancock V, Klemm P. 2008. Inactivation of efflux pumps abolishes bacterial biofilm formation. Appl Environ Microbiol 74:7376–7382. doi: 10.1128/AEM.01310-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pickering BS, Smith DR, Watnick PI. 2012. Glucose-specific enzyme IIA has unique binding partners in the Vibrio cholerae biofilm. mBio 3:e00228–. doi: 10.1128/mBio.00228-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Houot L, Watnick PI. 2008. A novel role for enzyme I of the Vibrio cholerae phosphoenolpyruvate phosphotransferase system in regulation of growth in a biofilm. J Bacteriol 190:311–320. doi: 10.1128/JB.01410-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pan Y, Breidt F Jr, Gorski L. 2010. Synergistic effects of sodium chloride, glucose, and temperature on biofilm formation by Listeria monocytogenes serotype 1/2a and 4b strains. Appl Environ Microbiol 76:1433–1441. doi: 10.1128/AEM.02185-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hung RJ, Chien HS, Lin RZ, Lin CT, Vatsyayan J, Peng HL, Chang HY. 2007. Comparative analysis of two UDP-glucose dehydrogenases in Pseudomonas aeruginosa PAO1. J Biol Chem 282:17738–17748. doi: 10.1074/jbc.M701824200. [DOI] [PubMed] [Google Scholar]
- 55.Nucleo E, Steffanoni L, Fugazza G, Migliavacca R, Giacobone E, Navarra A, Pagani L, Landini P. 2009. Growth in glucose-based medium and exposure to subinhibitory concentrations of imipenem induce biofilm formation in a multidrug-resistant clinical isolate of Acinetobacter baumannii. BMC Microbiol 9:270. doi: 10.1186/1471-2180-9-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Waldrop R, McLaren A, Calara F, McLemore R. 2014. Biofilm growth has a threshold response to glucose in vitro. Clin Orthop Relat Res 472:3305–3310. doi: 10.1007/s11999-014-3538-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kamada N, Chen GY, Inohara N, Nunez G. 2013. Control of pathogens and pathobionts by the gut microbiota. Nat Immunol 14:685–690. doi: 10.1038/ni.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ashida H, Mimuro H, Sasakawa C. 2015. Shigella manipulates host immune responses by delivering effector proteins with specific roles. Front Immunol 6:219. doi: 10.3389/fimmu.2015.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rowe JL, Starnes GL, Chivers PT. 2005. Complex transcriptional control links NikABCDE-dependent nickel transport with hydrogenase expression in Escherichia coli. J Bacteriol 187:6317–6323. doi: 10.1128/JB.187.18.6317-6323.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang Y, Xiao M, Horiyama T, Zhang Y, Li X, Nishino K, Yan A. 2011. The multidrug efflux pump MdtEF protects against nitrosative damage during the anaerobic respiration in Escherichia coli. J Biol Chem 286:26576–26584. doi: 10.1074/jbc.M111.243261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim YJ, Yeo SG, Park JH, Ko HJ. 2014. Shigella vaccine development: prospective animal models and current status. Curr Pharm Biotechnol 14:903–912. doi: 10.2174/1389201014666131226123900. [DOI] [PubMed] [Google Scholar]
- 62.Kararli TT. 1995. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm Drug Dispos 16:351–380. doi: 10.1002/bdd.2510160502. [DOI] [PubMed] [Google Scholar]
- 63.Nickerson KP, McDonald C. 2012. Crohn's disease-associated adherent-invasive Escherichia coli adhesion is enhanced by exposure to the ubiquitous dietary polysaccharide maltodextrin. PLoS One 7:e52132. doi: 10.1371/journal.pone.0052132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schroeder A, Mueller O, Stocker S, Salowsky R, Leiber M, Gassmann M, Lightfoot S, Menzel W, Granzow M, Ragg T. 2006. The RIN: an RNA integrity number for assigning integrity values to RNA measurements. BMC Mol Biol 7:3. doi: 10.1186/1471-2199-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ewing B, Green P. 1998. Base-calling of automated sequencer traces using phred. II. Error probabilities. Genome Res 8:186–194. doi: 10.1101/gr.8.3.186. [DOI] [PubMed] [Google Scholar]
- 66.Ewing B, Hillier L, Wendl MC, Green P. 1998. Base-calling of automated sequencer traces using phred. I. Accuracy assessment. Genome Res 8:175–185. doi: 10.1101/gr.8.3.175. [DOI] [PubMed] [Google Scholar]
- 67.Hazen TH, Daugherty SC, Shetty A, Mahurkar AA, White O, Kaper JB, Rasko DA. 2015. RNA-Seq analysis of isolate- and growth phase-specific differences in the global transcriptomes of enteropathogenic Escherichia coli prototype isolates. Front Microbiol 6:569. doi: 10.3389/fmicb.2015.00569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Anders S, Huber W. 2010. Differential expression analysis for sequence count data. Genome Biol 11:R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kendall MM, Rasko DA, Sperandio V. 2010. The LysR-type regulator QseA regulates both characterized and putative virulence genes in enterohaemorrhagic Escherichia coli O157:H7. Mol Microbiol 76:1306–1321. doi: 10.1111/j.1365-2958.2010.07174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Faherty CS, Maurelli AT. 2009. Spa15 of Shigella flexneri is secreted through the type III secretion system and prevents staurosporine-induced apoptosis. Infect Immun 77:5281–5290. doi: 10.1128/IAI.00800-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Crabtree J, Agrawal S, Mahurkar A, Myers GS, Rasko DA, White O. 2014. Circleator: flexible circular visualization of genome-associated data with BioPerl and SVG. Bioinformatics 30:3125–3127. doi: 10.1093/bioinformatics/btu505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Oliveros JC. 2007–2015 Venny. An interactive tool for comparing lists with Venn's diagrams. http://bioinfogp.cnb.csic.es/tools/venny/.
- 73.Senerovic L, Tsunoda SP, Goosmann C, Brinkmann V, Zychlinsky A, Meissner F, Kolbe M. 2012. Spontaneous formation of IpaB ion channels in host cell membranes reveals how Shigella induces pyroptosis in macrophages. Cell Death Dis 3:e384. doi: 10.1038/cddis.2012.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Faherty CS, Wu T, Morris CR, Grassel CL, Rasko DA, Harper JM, Shea-Donohue T, Fasano A, Barry EM. 2016. The synthesis of OspD3 (ShET2) in Shigella flexneri is independent of OspC1. Gut Microbes 7:486–502. doi: 10.1080/19490976.2016.1239682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Arizmendi O, Picking WD, Picking WL. 2016. Macrophage apoptosis triggered by IpaD from Shigella flexneri. Infect Immun 84:1857–1865. doi: 10.1128/IAI.01483-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Formal SB, Dammin GJ, Labrec EH, Schneider H. 1958. Experimental Shigella infections: characteristics of a fatal infection produced in guinea pigs. J Bacteriol 75:604–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sansonetti PJ, Kopecko DJ, Formal SB. 1982. Involvement of a plasmid in the invasive ability of Shigella flexneri. Infect Immun 35:852–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fiorentino M, Levine MM, Sztein MB, Fasano A. 2014. Effect of wild-type Shigella species and attenuated Shigella vaccine candidates on small intestinal barrier function, antigen trafficking, and cytokine release. PLoS One 9:e85211. doi: 10.1371/journal.pone.0085211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hanahan D. 1983. Studies on transformation of Escherichia coli with plasmids. J Mol Biol 166:557–580. doi: 10.1016/S0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 80.McCormick BA, Franklin DP, Laux DC, Cohen PS. 1989. Type 1 pili are not necessary for colonization of the streptomycin-treated mouse large intestine by type 1-piliated Escherichia coli F-18 and E. coli K-12. Infect Immun 57:3022–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Levine MM, Bergquist EJ, Nalin DR, Waterman DH, Hornick RB, Young CR, Sotman S. 1978. Escherichia coli strains that cause diarrhoea but do not produce heat-labile or heat-stable enterotoxins and are non-invasive. Lancet i:1119–1122. [DOI] [PubMed] [Google Scholar]
- 82.Casadaban MJ. 1976. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol 104:541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- 83.Hurley BP, Thorpe CM, Acheson DW. 2001. Shiga toxin translocation across intestinal epithelial cells is enhanced by neutrophil transmigration. Infect Immun 69:6148–6155. doi: 10.1128/IAI.69.10.6148-6155.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jandhyala DM, Rogers TJ, Kane A, Paton AW, Paton JC, Thorpe CM. 2010. Shiga toxin 2 and flagellin from shiga-toxigenic Escherichia coli superinduce interleukin-8 through synergistic effects on host stress-activated protein kinase activation. Infect Immun 78:2984–2994. doi: 10.1128/IAI.00383-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nataro JP, Deng Y, Cookson S, Cravioto A, Savarino SJ, Guers LD, Levine MM, Tacket CO. 1995. Heterogeneity of enteroaggregative Escherichia coli virulence demonstrated in volunteers. J Infect Dis 171:465–468. doi: 10.1093/infdis/171.2.465. [DOI] [PubMed] [Google Scholar]
- 86.Czeczulin JR, Balepur S, Hicks S, Phillips A, Hall R, Kothary MH, Navarro-Garcia F, Nataro JP. 1997. Aggregative adherence fimbria II, a second fimbrial antigen mediating aggregative adherence in enteroaggregative Escherichia coli. Infect Immun 65:4135–4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fiorentino M, Lammers KM, Levine MM, Sztein MB, Fasano A. 2013. In vitro intestinal mucosal epithelial responses to wild-type Salmonella typhi and attenuated typhoid vaccines. Front Immunol 4:17. doi: 10.3389/fimmu.2013.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cherayil BJ, McCormick BA, Bosley J. 2000. Salmonella enterica serovar typhimurium-dependent regulation of inducible nitric oxide synthase expression in macrophages by invasins SipB, SipC, and SipD and effector SopE2. Infect Immun 68:5567–5574. doi: 10.1128/IAI.68.10.5567-5574.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yanisch-Perron C, Vieira J, Messing J. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.