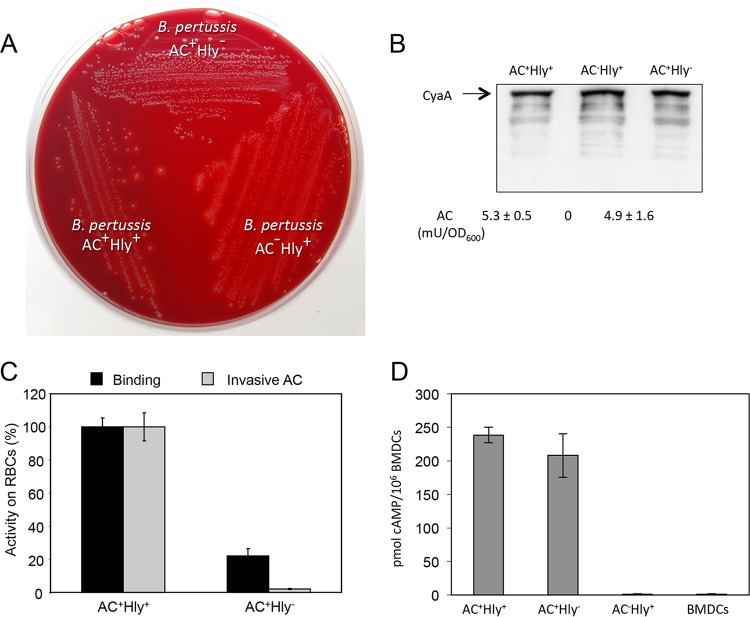
FIG 1.
The AC+ Hly− variant of CyaA is produced at normal levels and preserves the DC-binding and cAMP-elevating activity of intact CyaA. (A) Parental AC+ Hly+ B. pertussis, the nonhemolytic mutant expressing the CyaA-E570Q+K860R toxin (AC+ Hly−), and the AC− Hly+ mutant producing an enzymatically inactive CyaA-AC− toxoid were grown on Bordet-Gengou agar with 15% defibrinated sheep blood for 5 days at 37°C. (B) Bacteria were grown in liquid Stainer-Scholte (SS) medium for 18 h at 37°C, and CyaA toxin was detected on Western blots of bacterial lysates using the anti-RTX 9D4 antibody. Values below the blot indicate the total AC enzyme activities of CyaA associated with the outer surface of bacterial cells grown in SS medium with 0.13 mM Ca2+ ions. (C) CD11b− sheep erythrocytes (5 × 108/ml) were incubated at 37°C with bacterial lysates (50 ng CyaA/ml). After 30 min, aliquots were taken for determination of the cell-associated AC activity (binding) and of the AC activity internalized into erythrocytes and protected against digestion by externally added trypsin (invasive AC). Activities are expressed as percentages of intact CyaA activity and represent average values ± standard deviations from two independent determinations performed in duplicate. (D) AC domain translocation was assessed by determining the intracellular concentration of cAMP generated in cells following incubation with diluted bacterial lysates (final CyaA concentration of 7.5 ng/ml). The results represent the average of values obtained in at least two independent experiments performed in duplicate.