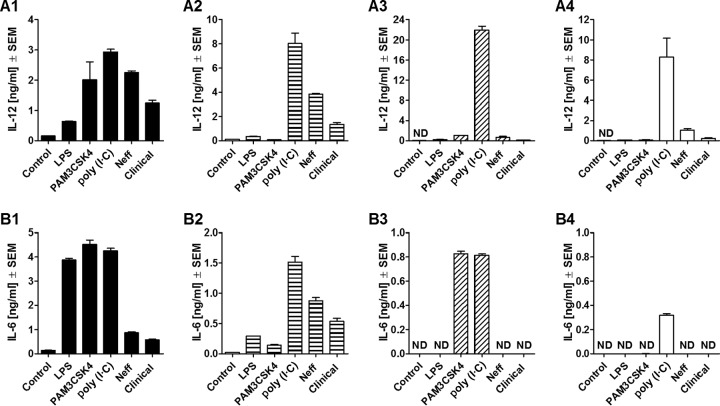
FIG 5.
Release of IL-12 (A) and IL-6 (B) by comparison of C57BL/6 WT (A1 and B1) and C57BL/6 TLR2−/− (A2 and B2), TLR4−/− (A3 and B3), and TLR2/4−/− (A4 and B4) mouse macrophages at 24 h after coincubation with Acanthamoeba trophozoites. Murine macrophages (1 × 106) obtained from C57BL/6 WT and knockout mice were challenged with 1 × 106 trophozoites of either the A. castellanii Neff strain (Neff) or the clinical isolate (Clinical). LPS, PAM3CSK4, and poly(I·C) at 200 ng/ml, 320 ng/ml, and 10 μg/ml, respectively, were used as positive controls to stimulate macrophages. Uninfected macrophages (Control) were considered the negative control. The experiment was performed twice. Results represent the mean ± standard error (n = 3). Student's t test was applied to evaluate differences between C57BL/6 WT mice and the C57BL/6 TLR2−/−, TLR4−/−, and TLR2/4−/− mice. Values below the detectable levels are indicated in the graphs as ND (not detected). IL-12 and IL-6 production under trophozoite/TLR2−/− macrophage coincubation conditions was not significantly diminished in comparison with that under trophozoite/WT macrophage coincubation conditions, suggesting that the production of these proinflammatory cytokines by macrophages in response to Acanthamoeba is not TLR2 dependent. On the other hand, in the absence of TLR4, trophozoite-induced IL-12 production by macrophages was significantly decreased, whereas IL-6 production was completely ablated. The same pattern was observed when neither TLR2 nor TLR4 was expressed. Therefore, TLR4 appeared to be the main TLR involved in the recognition of and response to A. castellanii.