Figure 2.
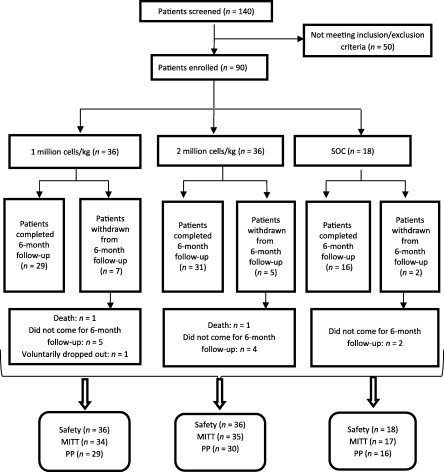
Total number of patients screened, allocated in each arm, followed up, and analyzed in the trial. Diagram as per the Consolidated Standards for Reporting Trials (CONSORT) flowchart. MITT population refers to all patients who received Stempeucel and had at least one rest‐pain assessment at visit 4. PP population refers to patients who had both the baseline and visit 6 rest‐pain assessments, with no major protocol deviation. Abbreviations: MITT, modified intention‐to‐treat; PP per protocol; SOC, standard of care.
