Figure 6.
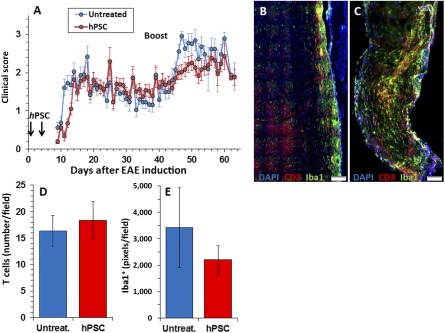
Intramuscular hPSC injection before EAE induction delayed disease onset and attenuated relapse; 2 × 106 hPSCs were injected i.m. on days 1 and 5 after EAE induction before the onset of clinical signs. The clinical scores of all mice participating in this project (n = 42 implanted and 45 control mice, part of which were sacrificed at different time points for pathological and immunological studies) were analyzed. (A): A mild, but statistically significant, postponement of disease onset by 2 days was observed in i.m. implanted mice (p = .026) followed by a comparable disease course. In the postacute phase, no significant difference was found between the groups. To evaluate whether i.m. hPSC treatment exerted long‐term therapeutic effects, a relapse was induced on day 40. Relapse was observed in 85% of the control versus 54% of the hPSC‐treated mice (p < .05). The intensity of the relapse was 2.32 ± 1 in the control group versus 1.51 ± 0.43 in the hPSC‐injected mice (p < .0005). By day 60, these differences were annulled, and the two experimental groups were identical clinically. Quantification of the inflammatory response in the spinal cord at this time showed no difference in the number of CD3+ T cells, with a mild nonsignificant decline in Iba1+ macrophage/microglia in the hPSC‐treated (n = 16; C–E) versus control (n = 13; B, D, E) mice. Scale bars = 100 µm (B, C). Abbreviations: DAPI, 4′,6‐diamidino‐2‐phenylindole; EAE, experimental autoimmune encephalomyelitis; hPSC, human placental stromal cell; Untreat., untreated.
