Abstract
The objective is to investigate whether human amniotic fluid stem cells (hAFSCs) grafting into the bladder may influence bladder functional and molecular changes in an animal stroke model. Female rats were divided into three groups: sham, middle cerebral artery occlusion (MCAO) alone, and MCAO plus 1 × 106 hAFSCs transplanting into bladder wall. Bladder function was analyzed by cystometry at days 3 and 10 after MCAO. The expressions of bladder nerve growth factor (NGF), M2‐muscarinic, M3‐muscarinic, and P2X1 receptors were measured by immunohistochemistry and real‐time polymerase chain reaction. When compared with sham‐operated group, MCAO alone rats had significant increase in residual volume and decrease in voided volume and intercontraction interval; however, these bladder dysfunctions were improved following hAFSCs transplantation. The immunoreactivities of NGF, M3, and P2X1 significantly decreased at days 3 and 10, but M2 increased at day 3 after MCAO. Following hAFSCs transplantation, the immunoreactivities of NGF and P2X1 significantly increased at day 3, and M2 increased at day 10 after MCAO. The mRNAs of NGF, M2, and M3 significantly increased at day 3, but NGF and M2 decreased at day 10 after MCAO. Following hAFSCs transplantation, there was significant decrease in M2 mRNA at day 3 and increase in P2X1 mRNA at days 3 and 10 after MCAO. Bladder dysfunction caused by MCAO can be improved by hAFSCs transplanting into bladder which may be related to the expressions of bladder NGF, and muscarinic and P2X1 receptors. Stem Cells Translational Medicine 2017;6:1227–1236
Keywords: Amniotic fluid stem cell, Bladder, Cystometry, Middle cerebral artery occlusion, Stroke
Significance Statement.
This is the first in the literature using human amniotic fluid stem cells for treatment in a rat model of bladder dysfunction. Bladder dysfunction caused by middle cerebrate artery occlusion can be improved by amniotic fluid stem cells transplanting directly into bladder that may be related to the expressions of bladder nerve growth factor, muscarinic and P2X1 receptors
Introduction
Cerebral stroke is ranked as one of the leading causes of death, and the poststroke neurological injury is the most important problem to cause disability worldwide. In addition to neurological disability, voiding dysfunction is common in the patients with stroke and impairs life quality severely in the long term. Bladder dysfunction related to stroke is characterized by a plethora of urologic symptoms, such as overactive bladder, voiding difficulty, and urinary incontinence 1, 2. Neurogenic overactive bladder after cerebral stroke is currently treated with antimuscarinic therapy, detrusor botulinum toxin injection, or sacral neuromodulation; however, these therapeutic methods are not effective and associated with adverse effects 3. Recently, we have reported that systemic administration of human umbilical cord blood CD34 cells to middle cerebral artery occlusion (MCAO) rats could significantly reduce infarct volume 4. Treatment with amniotic fluid stem cells (AFSCs) transplantation may facilitate functional recovery in a rodent model of ischemic stroke 5, 6. Therefore, stem cell transplantation seems a promising treatment for bladder dysfunction after cerebral stroke.
Bladder overactivity induced by MCAO in rats has been found to involve receptors in the brain such as dopamine and glutamate 7. Nerve growth factor (NGF) and muscarinic receptors in cerebral‐infarcted rats are also involved in the regulation of the micturition reflex in central and peripheral nervous systems 8, 9, 10, 11. NGF has an effect on bladder dysfunction by mediating morphological and functional changes in sensory neurons innervating the bladder 10. A significant increase in the density of muscarinic receptors has been found in the urinary bladder after cerebral infarction in rats 9. In the bladder of animals, there is parasympathetic excitatory cotransmission with the cholinergic and purinergic components acting via muscarinic and P2X1 receptors, respectively, 12.
At present, no study has ever been designed to demonstrate the effect of AFSCs on bladder dysfunction induced by cerebral ischemia in animals. The present study was conducted to investigate whether human amniotic fluid stem cells (hAFSCs) grafting into the bladder wall may influence bladder functional and molecular changes in a rat stroke model. Since it is reported that local injection of stem cells may ameliorate impaired detrusor contractility of injured bladder in rodent models 13, 14, and local injection may cause more stem cells in the submucosal connective tissue and muscular tissue of bladder than intravenous injection 15, so we used local injection into bladder in the present study.
Materials and Methods
Animal Model
All protocols were approved by the Institutional Ethics Committee for the Care and Use of Experimental Animals and the Institutional Review Board of our hospital. Female Sprague Dawley rats were maintained at 21°C–23°C room temperatures and 47% humidity with a 12‐hour light‐dark cycle and free access to standard laboratory chow and tap water. Rats (270–320 g) were assigned into 3 groups: (a) sham‐operated group (n = 10): injection with 0.3 ml phosphate buffered saline (PBS) at 3 hours after sham operation, (b) MCAO alone rats with no hAFSCs treatment (n = 10): 0.3 ml PBS injection at 3 hours after MCAO, (c) MCAO rats with hAFSCs treatment (n = 10): injection of 1 × 106 hAFSCs cells in 0.3 ml PBS at 3 hours after MCAO. Bladder function was analyzed using conscious cystometry at days 3 and 10 after MCAO. Expressions of NGF, M2‐muscarinic receptor (M2), M3‐muscarinic receptor (M3), and P2X1 were measured by immunohistochemistry, and real‐time polymerase chain reaction. The schema of the experimental procedure is present in Supporting Information Figure 1.
Focal Cerebral Ischemia Model
The left MCAO was used as an acute ischemic model according to previously reported method 16, 17. Under isoflurane general anesthesia, the proximal portion of external carotid artery was tightly ligated with a silk suture. A 20‐mm 4‐0 nylon surgical thread was inserted from left external carotid artery into internal carotid artery to occlude the middle cerebral artery. Left common carotid artery was then permanently ligated, and the wound was temporarily closed. Anesthesia was discontinued after these procedures were completed. After a 60‐minutes occlusion of left middle cerebral artery, rat was anesthetized again, and the wound was opened to remove nylon surgical thread to recanalize middle cerebral artery. In the sham‐operated group, similar procedures were conducted except the ligation or occlusion of any cerebral vessel. Endovascular suture occlusion of the middle cerebral artery for 60 minutes could result in irreversible cerebral ischemic injury in both the cerebral cortex and striatum. We only included animals that exhibited neurological evidence of right‐side weakness with upper‐limb dominance.
Isolation and Characterization of hAFSC for Transplantation
The hAFSCs were obtained from the freshly collected amniotic fluid by routine amniocentesis from healthy pregnant donors with 15–20 gestational weeks. Cells were cultured in StemPro MSC SFM (serum free medium) supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA) and incubated at 37°C with 5% carbon dioxide. Culture medium was changed every 3–4 days. The specific surface antigens of hAFSCs were characterized by flow cytometry analyses as shown in our previous work 18. The cells in culture were trypsinized and stained with phycoerythrin (PE)‐conjugated antibodies against CD44, CD73, CD90, CD105, CD117, and CD45 (BD PharMingen, CA). Thereafter, the cells were analyzed using the Calibur flow cytometer (Becton Dickinson, Heidelberg, Germany). Passage 6‐8 hAFSCs were collected and prepared to a final concentration of 1 × 106 cells per 0.3 milliliter in PBS. In the hAFSCs‐treated groups, 1 × 106 collected hAFSCs were transplanted into each rat at 3 hours after MCAO by injection into the five sites of bladder (anterior, posterior, bilateral, and dome) under inhalation anesthesia. Before each local injection, the syringe was pushed backwards to confirm the needle was not present inside the vessel.
Cystometric Study
All rats received suprapubic tube implantation under isoflurane general anesthesia 3 days prior to implementation of cystometry. The animals were placed in special metabolic cages (Med Associates, Saint Albans, VT) to perform conscious cystometric studies at days 3 and 10 after MCAO according to the methods described in our previous study 19. Briefly, the suprapubic catheter was connected to both the syringe pump and the pressure transducer. Pressure and force transducer signals were amplified, recorded on a chart recorder and digitized for computer data collection. The bladder was then filled with room‐temperature 0.9% saline at 5 ml/hour through the bladder catheter, while bladder pressure was recorded. Urine was collected in a beaker on a balance placed beneath each cage. Changes in the weight of the collection were recorded. Saline infusion was continued until rhythmic bladder micturition contractions became stable. All of the cystometric parameters on five representative micturition cycles were collected for analyzing, including peak voiding pressure, intercontraction interval, voided volume, and residual volume. Cystometry Analysis Version 1.05 (Catamount Research and Development, Saint Albans, VT) was used for cystometric analysis.
Immunohistochemistry
Animals were euthanized after cystometry, the dissected bladders were fixed in an optimal cutting temperature compound, frozen in powdered dry ice and stored at −80°C. The bladders were then subjected to cryosection (10 μm) at −18°C. Immunostaining against NGF, M2, M3, and P2X1 in fresh‐frozen bladder sections was performed with an avidin‐biotin peroxidase method. First, fresh‐frozen sections were fixed in acetone 10 minutes for NGF and 4% paraformaldehyde 10 minutes for M2, M3, and P2X1, air dried and then rinsed with PBS. After blocking with Dako REAL peroxidase blocking solution (code S2023, DAKO Corp, Carpinteria, CA) for 20 minutes, sections were washed and incubated for 18–20 hours at 4°C with a rabbit polyclonal antibody directed against NGF (1:750, OriGene Technologies, Inc.), M2 (1:1,000, Millipore, Temecula, CA), M3 (1:1,000, Santa Cruz Biotechnology, Santa Cruz, CA) and P2X1 (1:500, Santa Cruz Biotechnology, Santa Cruz, CA). Then, sections were washed and incubated for one hour using biotinylated secondary antibodies at a 1:500 dilution (Vector Laboratories, Burlingame, CA). Staining was developed with 3,3′‐diaminobenzidine plus hydrogen peroxide as the chromogen. The ratio of the optical density of MCAO rats with or without hAFSCs treatment to that of sham‐operated rat was determined for NGF, M2, M3, and P2X1 analyses. Image‐Pro Plus Software (Media Cybernetics, Silver Spring, MD) was used for immunoreactivity measurement.
Immunofluorescence
Immunofluorescent staining was done according to the previous report 20. The fresh‐frozen bladder sections were first fixed with precooled acetone for 10 minutes, and then washed in PBS three times for 5 minutes. The sections were blocked with 10% fetal bovine serum in PBS for 20 minutes at room temperature and then incubated with rabbit polyclonal antibody (PE) directed against human PAX7 (1:50, LifeSpan BioSciences, Seattle, WA) in a humidified chamber overnight at 4°C. The sections were stained with donkey anti‐rabbit IgG antibodies (1:500, Santa Cruz Biotechnology, Santa Cruz, CA) for 45 minutes and then stained with 4',6‐diamidino‐2‐phenylindole (DAPI) (Santa Cruz Biotechnology, Santa Cruz, CA). All images were detected by confocal microscope (Leica, Wetzlar, Germany).
Real‐Time Polymerase Chain Reaction
Real‐time PCR was carried out according to the manufacturer's protocol. Total RNAs were prepared using a Trizol reagent (Invitrogen, Carlsbad, CA) and incubated in reverse transcription mixture at 25°C for 5 minutes, 50°C for 1 hour, 70°C for 15 minutes; finally, the tubes were cooled to 4°C for 5 minutes. Gene expression for NGF, M2, M3, and P2X1 in the bladder tissue was analyzed by real‐time PCR using inventoried TaqMan assays from Applied Biosystems (Life Technologies, Grand Island, NY). The codes for NGF, M2, M3, and P2X1 assays were Rn01533872‐m1, Rn02532311‐s1, Rn00560986‐s1, and Rn00564454‐m1, respectively, (Applied Biosystems). GAPDH assays codes (Rn99999916‐s1) were used as an endogenous control to allow for quantification of relative gene expression. Thermal cycling and fluorescence detection were performed using an ABI Prism 7900HT Sequence Detection System (Applied Biosystems). PCR conditions were 50°C for 2 minutes, 95°C for 10 minutes, followed by 40 cycles at 95°C for 15 seconds and 60°C for one minute. The data were calculated using the 2[‐Delta Delta C(T)] method 21. A ratio of the mRNA level of ischemic rats with or without hAFSCs treatment to that of sham‐operated rats was determined. The values were summated and expressed as mean ± SD and were compared statistically among sham operation and each time point in MCAO group.
Statistical Analysis
The data were analyzed statistically using one‐way analysis of variance test followed by a Tukey test. Values were considered significant at p < .05. Prism 5 software for statistical analysis (GraphPad, San Diego, CA) was used for all data.
Results
Characterization of hAFSC Showing the Stem Cell Markers
We successfully obtained hAFSC as previously described by our group and De Coppi et al. 18, 22 with expressions of CD117 (stem cell markers), CD44 (cell migration marker), and mesenchymal stem cell markers including CD73, CD90, and CD105. These cells did not express CD45 (hematopoietic stem cell marker) (Supporting Information Fig. 2).
Bladder Overactivity Subsequent to MCAO Are Ameliorated by hAFSCs
A total of 6 rats were excluded, including no right‐side weakness in 3, death within 2 days after operation in 2, and death during operation in 1. Data of 60 rats were collected and the final number in each group was kept at 10 for statistical analysis.
When compared with sham‐operated rats, cystometric studies showed that MCAO alone rats had significant increase in residual volume and decrease in voided volume and intercontraction interval (all p < .05) but no difference in peak voiding pressure at days 3 and 10 after MCAO. When compared with MCAO alone group, hAFSCs group had less residual volume, more voided volume and longer intercontraction interval at days 3 and 10 after MCAO (all p < .05; Fig. 1).
Figure 1.
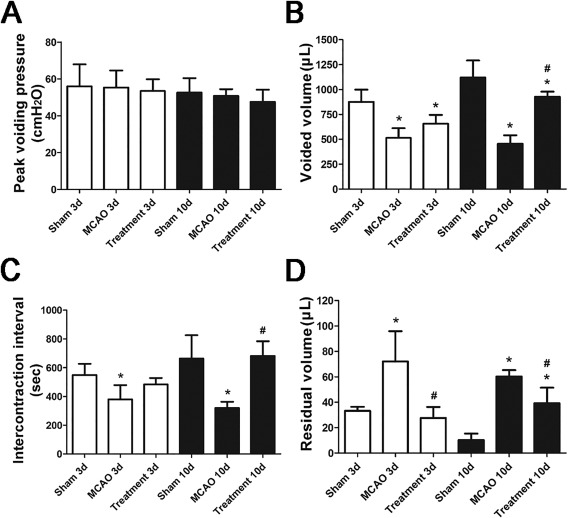
Urodynamic studies for all groups before and after treatment. Cystometric results in the experimental rats (A–D) are presented (n = 10). MCAO rats show no change in peak voiding pressure (A), but a significant decrease in voided volume (B) and intercontraction interval (C), and an increase in residual volume (D) at days 3 and 10 after MCAO. However, these bladder dysfunctions can be improved following human amniotic fluid stem cells transplantation. *Compared to sham‐operated group, p < .05. # Compared to MCAO alone group, p <.05. Abbreviation: MCAO, middle cerebral artery occlusion.
Expressions of NGF, P2X1, and Muscarinic Immunoreactivity and Human PAX7 in Bladder After MCAO
When compared with sham‐operated group, MCAO alone rats had significant decrease in the immunoreactivities of NGF, M3 and P2X1 at days 3 and 10 after MCAO (all p < .05); however, M2 immunoreactivity transiently increased at day 3 (p < .05) and then decreased at day 10 after MCAO (p < .05). When compared with MCAO alone group, hAFSCs group had significant increase of NGF and P2X1 immunoreactivities at day 3, and increase of M2 at day 10 after MCAO (all p < .05, Figs. 2, 3, 4, 5). The immunofluorescence of human PAX7 was not seen in the bladder wall of sham‐operated rat, but could be seen in that of hAFSCs‐treated rat (Supporting Information Fig. 3).
Figure 2.
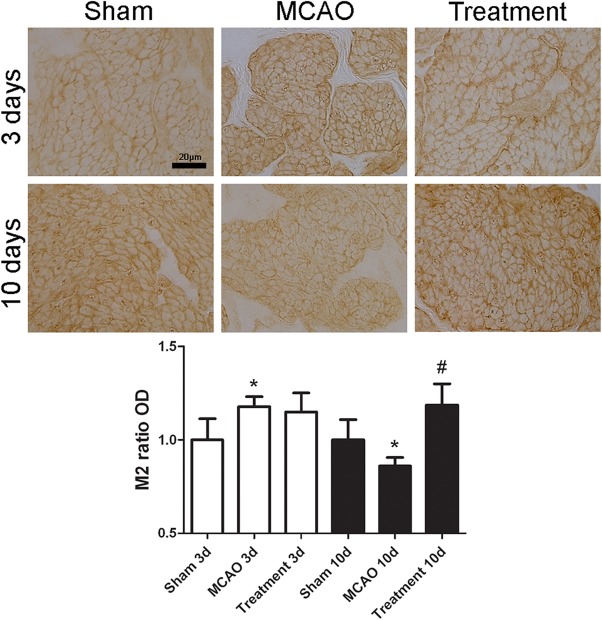
Temporal expressions of bladder M2 immunoreactivity in MCAO rats (n = 10). Expressions of M2 immunoreactivity significantly increase at day 3 but decrease at day 10 after MCAO. Following human amniotic fluid stem cells transplantation, the immunoreactivity of M2 significantly increases at day 10 after MCAO. Bar indicates 20 μm. *Compared to sham‐operated group, p <.05. # Compared to MCAO alone group, p < .05. Abbreviation: MCAO, middle cerebral artery occlusion.
Figure 3.
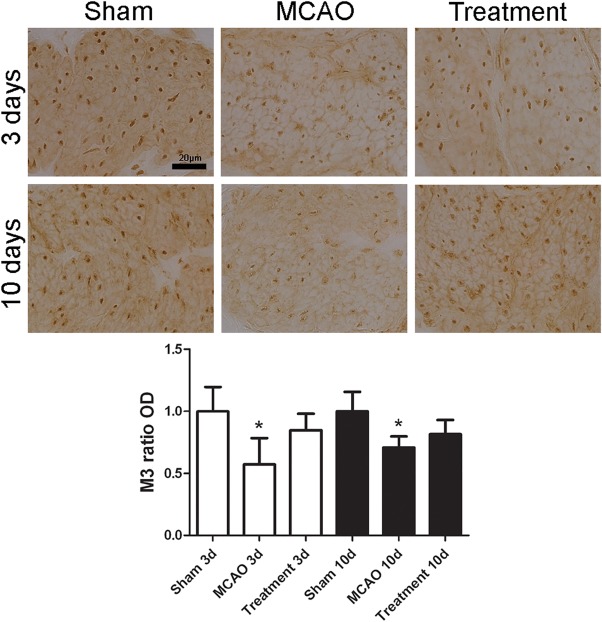
Temporal expressions of bladder M3 immunoreactivity in MCAO rats (n = 10). Expressions of M3 immunoreactivity significantly decrease at days 3 and 10 after MCAO. However, there is no significant change after human amniotic fluid stem cells transplantation. Bar indicates 20 μm. *Compared to sham‐operated group, p < .05. Abbreviation: MCAO, middle cerebral artery occlusion.
Figure 4.

Temporal expressions of bladder NGF immunoreactivity in MCAO rats (n = 10). Expressions of NGF immunoreactivity significantly decrease at days 3 and 10 after MCAO. Following human amniotic fluid stem cells transplantation, the NGF immunoreactivity significantly increases at day 3 after MCAO. Bar indicates 20 μm. *Compared to sham‐operated group, p < .05. # Compared to MCAO alone group, p < .05. Abbreviations: MCAO, middle cerebral artery occlusion; NGF, nerve growth factor.
Figure 5.
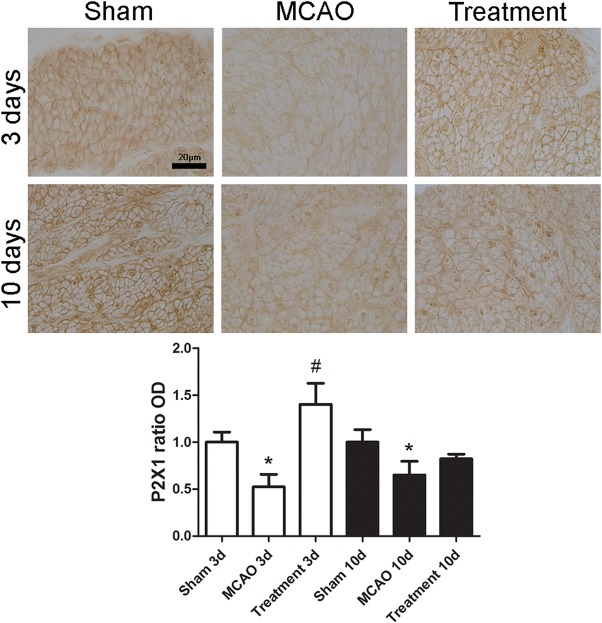
Temporal expressions of bladder P2X1 immunoreactivity in MCAO rats (n = 10). Expressions of P2X1 immunoreactivity significantly decrease at days 3 and 10 after MCAO. Following human amniotic fluid stem cells transplantation, the immunoreactivity of P2X1 significantly increases at day 3 after MCAO. Bar indicates 20 μm. *Compared to sham‐operated group, p < .05. # Compared to MCAO alone group, p < .05. Abbreviation: MCAO, middle cerebral artery occlusion.
Expressions of NGF, M2, and M3 mRNA in Bladder After MCAO
In Figure 6, when compared with sham‐operated group, MCAO alone rats had significant increase of NGF, M2, and M3 mRNA at day 3 (all p < .05), but decrease of M2 at day 10 after MCAO (p < .05). When compared with MCAO alone group, hAFSCs group had significant decrease of M2 mRNA at day 3 and increase of P2X1 mRNA at days 3 and 10 after MCAO (all p < .05).
Figure 6.
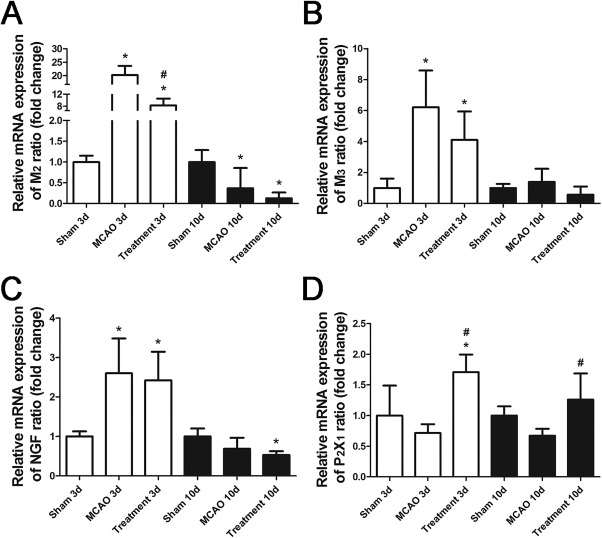
Comparison of mRNA expression level before and after treatment. Relative mRNA expressions of M2 (A), M3 (B), NGF (C), and P2X1 (D) after MCAO. The NGF, M2, and M3 mRNAs significantly increase at day 3, but NGF and M2 decrease at day 10 after MCAO. Following human amniotic fluid stem cells transplantation, there is significant decrease in M2 mRNA at day 3 and increase in P2X1 after MCAO. Bar indicates 20 μm. *Compared to sham‐operated group, p < .05. # Compared to MCAO alone group, p < .05. Abbreviations: MCAO, middle cerebral artery occlusion; NGF, nerve growth factor.
Discussion
Previous clinical reports have shown that bladder dysfunction may occur in the acute stage of stroke 23, 24, and around 70% ischemic stroke patients have detrusor overactivity 25. Animal study also demonstrated that after MCAO, bladder capacity and voided volume were significantly lower in the ischemic than in the sham rats, which was suggested due to detrusor overactivity 9. The mechanism of reduced bladder capacity in ischemic rats is supposed due to neuronal damage in the forebrain with interruption of tonic inhibitory neuronal pathways toward the pontine micturition center that regulates parasympathetic tone to urinary bladder 26. The reduced parasympathetic tone may induce compensatory upregulation of muscarinic receptors 9.
In the present study, the ischemic rats showed decrease in voided volume and intercontraction interval and increase in residual volume after MCAO, indicating bladder overactivity. After MCAO, there was a compensatory upregulation of M2 immunoreactivity transiently at day 3 but both M2 and M3 reduced at day 10. There was also a compensatory upregulation of both M2 and M3 mRNAs at day 3 but M2 then reduced and M3 was back to sham level at day 10. This expression disparity between protein and mRNA may be related to the severity of bladder injury. The direct injection of hAFSCs to bladder wall delayed the compensatory upregulation of M2 immunoreactivity from day 3 to day 10 and recovered the M2 mRNA at day 10. There was also recovery of M3 immunoreactivity and mRNA to sham level from day 3 to day 10 compared to MCAO alone. Similarly, after hAFSCs treatment, NGF immunoreactivity upregulated transiently at day 3 but recovered at day 10 and no change of mRNA. The P2X1 mRNA and immunoreactivity increased at day 3 and P2X1 immunoreactivity recovered at day 10. These data suggest muscarinic and P2X1 receptors and NGF are recovered mostly to sham level at day 10 except M2 and NGF mRNAs, which correlates well to the recovery of bladder function to sham level at day 10. The summary of NGF, M2, M3 and P2X1 expressions in bladder after MCAO is presented in Figure 7.
Figure 7.
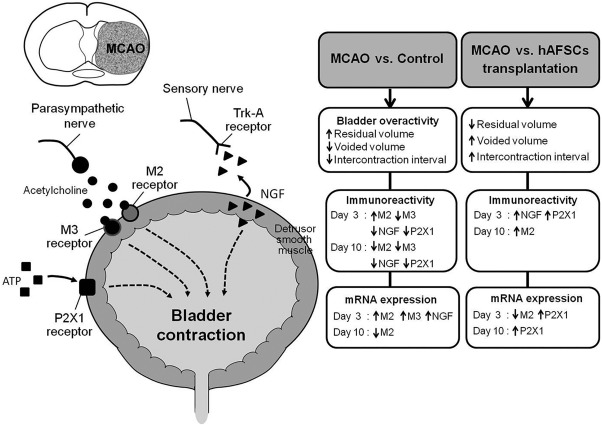
Summary of NGF, M2, M3, and P2X1 expressions in bladder after MCAO. Schematic diagram of the peripheral neurotransmitters and NGF responsible for bladder contraction. The mechanism of bladder overactivity induced by MCAO in rats has been found to involve muscarinic receptors (M2, M3), P2X1 receptor and NGF in the bladder. Bladder dysfunction can be improved following hAFSCs transplantation. When compared with MCAO alone group, hAFSCs transplantation can induce significant increase in the immunoreactivities of M2, P2X1, and NGF and significant increase in P2X1 mRNA but decrease in M2 mRNA. Abbreviations: hAFSCs, human amniotic fluid stem cells; MCAO, middle cerebral artery occlusion; nerve growth factor.
Bladder contraction mainly depends on the parasympathetic stimulation of the muscarinic receptors in the detrusor smooth muscle, but purinergic receptors also play a certain role in the contraction of bladder 27. Bladder purinergic activities are elicited predominantly by stimulation of the P2X1 receptors, and this purinoceptor subtype may be expressed in the rat and human bladders 28, 29. Purinergic receptors are activated by adenosine 5′‐triphosphate, which may have a more important role in bladder contraction in patients with overactive bladder 30. In a rat model, increased P2X1 receptor expression was suggested to contribute to the augmentation of bladder contractile response induced by hypoxia‐glucopenia and reoxygenation 31. A previous immunohistochemical study showed that P2X2 was elevated but P2X1 was significantly decreased by about 60% in the bladders of patients with detrusor overactivity 29. Similarly, our data revealed that when compared with sham‐operated rats, bladder P2X1 immunoreactivity was decreased at days 3 and 10 after MCAO, suggesting the involvement of P2X1 in the bladder activity following cerebral stroke.
NGF is normally present in bladder muscle cells and urothelium 32. NGF administered intramuscularly to the detrusor muscle can induce rat bladder hyperreflexia and neuronal hypersensitivity 33. Our previous study 19 found NGF immunoreactivity and mRNA in the bladder muscle declined significantly at days 7 and 28 after bilateral common carotid artery occlusion in rats. In the same study 19, we found that the increased NGF expression in bladder one day after cerebral hypoperfusion was associated with bladder hyperactivity which was found to sustain for a certain period even after NGF expression was decreased. Our present results were similar to our previous study 19 that the expressions of NGF protein and mRNA in bladder were decreased after MCAO, except the NGF mRNA increased transiently at day 3 after MCAO.
Our results demonstrated that bladder dysfunctions improved following bladder transplantation of hAFSCs in MCAO rats with less residual volume and improved voided volume and intercontraction interval. The hAFSCs can be obtained from amniotic fluid, grow easily in culture and appear phenotypically and genetically stable, supporting that these cells can act as a novel source for cell transplantation therapy 22, 34. In our previous studies, hAFSCs had been proved to have therapeutic effects in the mouse models of liver fibrosis and myocardial infarction 35, 36. Recently, these stem cells have also been used in various tissue repair studies, including bladder injury and neurologic disorders 13, 22. Soler et al. 34 reported that hAFSCs therapy may ameliorate bladder dysfunction in an animal model of Parkinson disease.
This study has some limitations. First, the present study did not demonstrate the therapeutic effect of hAFSCs on behavior recovery and infarct size after MCAO. However, the amount of hAFSCs needed to treat cerebral infarction through intravenous injection is much larger than that for local injection to treat bladder dysfunction. The present study used 1 × 106 cells per 0.3 milliliter injected in five bladder sites, which is much lower than the amount needed for intravenous injection. In such condition, there should be limited effect to the brain. Second, we had examined the effect of hAFSCs in different concentrations and found the concentration in this study was effective. However, it is possible that higher concentration may have better effect on micturition pressure after MCAO. Third, we only examined the effect of hAFSCs given at 3 hours after MCAO, because clinically available acute stroke treatment such as intravenous recombinant tissue plasminogen activator was used in a therapeutic time window within 3–4.5 hours 37. It is possible that hAFSCs could induce a better effect if given at other time points. Fourth, we used hAFSCs for the present study due to its low immunogenicity, low tumorigenesis, and anti‐inflammatory function 33, but xenogenic stem cell transplantation possibly involves host immune reactions. In the future study, these issues should be carefully examined. Fifth, it is possible the proteins we have examined might not be correlated very well with the bladder function. However, we found a relatively correlation of the recovery of muscarinic and P2X1 receptors and NGF with the recovery of bladder function at day 10. Previous reports also found that NGF can be regarded as a potential urinary biomarker for overactive bladder syndrome 38, and there is parasympathetic excitatory cotransmission with the cholinergic and purinergic components acting via muscarinic and P2X1 receptors, respectively, 12
Conclusion
In conclusion, our data support the inference that transplantation of hAFSCs can improve the bladder dysfunction following MCAO which may act through the regulation of bladder NGF, and muscarinic and P2X1 receptors.
Author Contributions
C.‐C.L., S.W.S.S., and Y.‐H.H.: conception and design, financial support, provision of study material or patients, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; Y.‐H.L. and T.‐H.L.: conception and design, financial support, provision of study material or patients, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest
Supporting information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Acknowledgments
This work was supported by Chang Gung Memorial Hospital Grant CMRPG3D0961, CMRPG3D1682, CMRPG3C056, CMRPG3D099, and Ministry of Science and Technology Taiwan Grant NSC103‐2314‐B‐182A‐101, MOST103‐2314‐B‐182A‐030, and MOST104‐2314‐B‐182A‐136.
References
- 1. Hall SA, Curto TM, Onyenwenyi A et al. Characteristics of persons with overactive bladder of presumed neurologic origin: Results from the Boston Area Community Health (BACH) survey. Neurourol Urodyn 2012;31:1149–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Linsenmeyer TA. Post‐CVA voiding dysfunctions: Clinical insights and literature review. NeuroRehabilitation 2012;30:1–7. [DOI] [PubMed] [Google Scholar]
- 3. Siddiqui NY, Wu JM, Amundsen CL. Efficacy and adverse events of sacral nerve stimulation for overactive bladder: A systematic review. Neurourol Urodyn 2010;29(suppl 1):S18–23. [DOI] [PubMed] [Google Scholar]
- 4. Liang CC, Liu HL, Chang SD et al. The Protective effect of human umbilical cord blood CD34+ cells and estradiol against focal cerebral ischemia in female ovariectomized rat: Cerebral MR imaging and immunohistochemical study. PLoS One 2016;11:e0147133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rehni AK, Singh N, Jaggi AS et al. Amniotic fluid derived stem cells ameliorate focal cerebral ischaemia‐reperfusion injury induced behavioural deficits in mice. Behav Brain Res 2007;183:95–100. [DOI] [PubMed] [Google Scholar]
- 6. Tajiri N, Acosta S, Glover LE et al. Intravenous grafts of amniotic fluid‐derived stem cells induce endogenous cell proliferation and attenuate behavioral deficits in ischemic stroke rats. PLoS One 2012;7:e43779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yokoyama O, Yoshiyama M, Namiki M et al. Glutamatergic and dopaminergic contributions to rat bladder hyperactivity after cerebral artery occlusion. Am J Physiol 1999;276:R935–942. [DOI] [PubMed] [Google Scholar]
- 8. Lee TH, Yang JT, Ko YS et al. Influence of ischemic preconditioning on levels of nerve growth factor, brain‐derived neurotrophic factor and their high‐affinity receptors in hippocampus following forebrain ischemia. Brain Res 2008;1187:1–11. [DOI] [PubMed] [Google Scholar]
- 9. Maruyama S, Kurosawa S, Takagi Y et al. Urodynamics and bladder muscarinic receptors in rats with cerebral infarction and bladder outlet obstruction. Neurosci Lett 2007;414:80–84. [DOI] [PubMed] [Google Scholar]
- 10. Ochodnicky P, Cruz CD, Yoshimura N et al. Nerve growth factor in bladder dysfunction: contributing factor, biomarker, and therapeutic target. Neurourol Urodyn 2011;30:1227–1241. [DOI] [PubMed] [Google Scholar]
- 11. Yokoyama O, Ootsuka N, Komatsu K et al. Forebrain muscarinic control of micturition reflex in rats. Neuropharmacology 2001;41:629–638. [DOI] [PubMed] [Google Scholar]
- 12. Burnstock G. Purinergic signalling in the urinary tract in health and disease. Purinergic Signal. 2014;10:103–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. De Coppi P, Callegari A, Chiavegato A et al. Amniotic fluid and bone marrow derived mesenchymal stem cells can be converted to smooth muscle cells in the cryo‐injured rat bladder and prevent compensatory hypertrophy of surviving smooth muscle cells. J Urol 2007;177:369–376. [DOI] [PubMed] [Google Scholar]
- 14. Lee JY, Piao S, Kim IG et al. Effect of human muscle‐derived stem cells on cryoinjured mouse bladder contractility. Urology 2012;80:224 e227–211. [DOI] [PubMed] [Google Scholar]
- 15. Huang YC, Shindel AW, Ning H et al. Adipose derived stem cells ameliorate hyperlipidemia associated detrusor overactivity in a rat model. J Urol 2010;183:1232–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee TH, Kato H, Chen ST et al. Expression of nerve growth factor and trkA after transient focal cerebral ischemia in rats. Stroke 1998;29:1687–1696; discussion 1697. [DOI] [PubMed] [Google Scholar]
- 17. Longa EZ, Weinstein PR, Carlson S et al. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1989;20:84–91. [DOI] [PubMed] [Google Scholar]
- 18. Soong YK, Huang SY, Yeh CH et al. The use of human amniotic fluid mesenchymal stem cells as the feeder layer to establish human embryonic stem cell lines. J Tissue Eng Regen Med 2015;9:E302–307. [DOI] [PubMed] [Google Scholar]
- 19. Liang CC, Lin YH, Liu HL et al. Bladder dysfunction induced by cerebral hypoperfusion after bilateral common carotid artery occlusion in rats. Neurourol Urodyn 2015;34:586–591. [DOI] [PubMed] [Google Scholar]
- 20. Piccoli M, Franzin C, Bertin E et al. Amniotic fluid stem cells restore the muscle cell niche in a HSA‐Cre, Smn(F7/F7) mouse model. Stem Cells 2012;30:1675–1684. [DOI] [PubMed] [Google Scholar]
- 21. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real‐time quantitative PCR and the 2(‐Delta Delta C(T)) Method. Methods 2001;25:402–408. [DOI] [PubMed] [Google Scholar]
- 22. De Coppi P, Bartsch G, Jr. , Siddiqui MM et al. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol 2007;25:100–106. [DOI] [PubMed] [Google Scholar]
- 23. Brittain KR, Peet SM, Castleden CM. Stroke and incontinence. Stroke 1998;29:524–528. [DOI] [PubMed] [Google Scholar]
- 24. Khan Z, Starer P, Yang WC et al. Analysis of voiding disorders in patients with cerebrovascular accidents. Urology 1990;35:265–270. [DOI] [PubMed] [Google Scholar]
- 25. Han KS, Heo SH, Lee SJ et al. Comparison of urodynamics between ischemic and hemorrhagic stroke patients; can we suggest the category of urinary dysfunction in patients with cerebrovascular accident according to type of stroke? Neurourol Urodyn 2010;29:387–390. [DOI] [PubMed] [Google Scholar]
- 26. Yokoyama O, Yoshiyama M, Namiki M et al. Role of the forebrain in bladder overactivity following cerebral infarction in the rat. Exp Neurol 2000;163:469–476. [DOI] [PubMed] [Google Scholar]
- 27. Zhang Q, Siroky M, Yang JH et al. Effects of ischemia and oxidative stress on bladder purinoceptors expression. Urology 2014;84:1249 e1241–1247. [DOI] [PubMed] [Google Scholar]
- 28. Aronsson P, Andersson M, Ericsson T et al. Assessment and characterization of purinergic contractions and relaxations in the rat urinary bladder. Basic Clin Pharmacol Toxicol 2010;107:603–613. [DOI] [PubMed] [Google Scholar]
- 29. O'Reilly BA, Kosaka AH, Knight GF et al. P2X receptors and their role in female idiopathic detrusor instability. J Urol 2002;167:157–164. [PubMed] [Google Scholar]
- 30. Ouslander JG. Management of overactive bladder. N Engl J Med 2004;350:786–799. [DOI] [PubMed] [Google Scholar]
- 31. Elliott RA, Tonnu A, Ghaffar N et al. Enhanced purinergic contractile responses and P2X1 receptor expression in detrusor muscle during cycles of hypoxia‐glucopenia and reoxygenation. Exp Physiol 2013;98:1683–1695. [DOI] [PubMed] [Google Scholar]
- 32. Steers WD, Tuttle JB. Mechanisms of Disease: The role of nerve growth factor in the pathophysiology of bladder disorders. Nat Clin Pract Urol 2006;3:101–110. [DOI] [PubMed] [Google Scholar]
- 33. Yoshimura N, Bennett NE, Hayashi Y et al. Bladder overactivity and hyperexcitability of bladder afferent neurons after intrathecal delivery of nerve growth factor in rats. J Neurosci 2006;26:10847–10855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Soler R, Fullhase C, Hanson A et al. Stem cell therapy ameliorates bladder dysfunction in an animal model of Parkinson disease. J Urol 2012;187:1491–1497. [DOI] [PubMed] [Google Scholar]
- 35. Peng SY, Chou CJ, Cheng PJ et al. Therapeutic potential of amniotic‐fluid‐derived stem cells on liver fibrosis model in mice. Taiwan J Obstet Gynecol 2014;53:151–157. [DOI] [PubMed] [Google Scholar]
- 36. Peng SY, Chou CJ, Cheng PJ et al. Intramuscular transplantation of pig amniotic fluid‐derived progenitor cells has therapeutic potential in a mouse model of myocardial infarction. Cell Transplant 2015;24:1003–1012. [DOI] [PubMed] [Google Scholar]
- 37. Lansberg MG, Bluhmki E, Thijs VN. Efficacy and safety of tissue plasminogen activator 3 to 4.5 hours after acute ischemic stroke: A metaanalysis. Stroke 2009;40:2438–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Seth JH, Sahai A, Khan MS et al. Nerve growth factor (NGF): A potential urinary biomarker for overactive bladder syndrome (OAB)? BJU Int 2013;111:372–380. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Supporting Information
Supporting Information


