Figure 7.
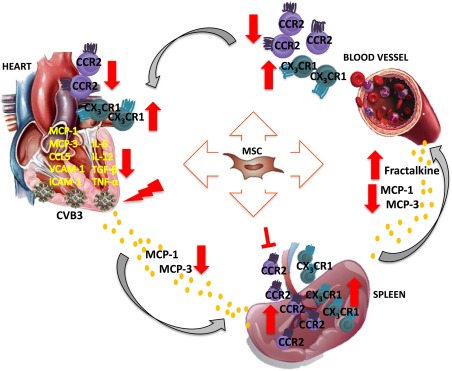
Hypothetical scheme how intravenous MSC application modulates monocytes trafficking to the heart in viral myocarditis. MSC application abrogated the cardiac expression of the chemokines MCP‐1, MCP‐3 and CCL5, which attract pro‐inflammatory monocytes, and lowered the levels of MCP‐1 and MCP‐3 in the circulation of CVB3‐infected mice. In the heart, MSC application further reduced the expression of the adhesion molecules VCAM‐1 and ICAM‐1 in CVB3 mice. These effects led to diminished trafficking and homing of pro‐inflammatory monocytes to the heart, as indicated by the reduced pro‐inflammatory monocytes in the blood and heart, as well as the downregulated expression of the pro‐inflammatory cytokines IL‐6, IL‐12, and TNF‐α and pro‐fibrotic TGF‐β in the heart of CVB3‐infected mice. In parallel, MSC injection increased systemic levels of fractalkine, raising the deployment of anti‐inflammatory monocytes to the heart. Finally, MSC retained the pro‐inflammatory monocyte subsets in the spleen, preventing their mobilization, emigration to the blood and accumulation in the heart, limiting further immune‐mediated cardiac injury and promoted spleen anti‐inflammatory monocytes. Abbreviations: CCR2, chemokine (C‐C motif) receptor‐2; CVB3, coxsackievirus B3; CX3CL1, chemokine (C‐X3‐C motif) ligand‐1; ICAM‐1, intercellular adhesion molecule‐1; IL, interleukin; MCP, monocyte chemotactic protein; MSC, mesenchymal stromal cell; TGF‐β1, transforming growth factor‐β1; TNF‐α, tumor necrosis factor‐α; VCAM‐1, vascular cell adhesion molecule‐1.
