Abstract
Staphylococcus aureus is an important pathogen responsible for a variety of diseases ranging from mild skin and soft tissue infections, food poisoning to highly serious diseases such as osteomyelitis, endocarditis, and toxic shock syndrome. Proper diagnosis of pathogen and virulence factors is important for providing timely intervention in the therapy. Owing to the invasive nature of infections and the limited treatment options due to rampant spread of antibiotic-resistant strains, the trend for development of vaccines and antibody therapy is increasing at rapid rate than development of new antibiotics. In this article, we have discussed elaborately about the host-pathogen interactions, clinical burden due to S aureus infections, status of diagnostic tools, and treatment options in terms of prophylaxis and therapy.
Keywords: Staphylococcus aureus, methicillin-resistant Staphylococcus aureus, MRSA, detection, antibiotic resistance, vaccine, antibody therapy
Introduction
Staphylococcus aureus is the leading cause of bacterial infections involving gastrointestinal, respiratory, skin and soft tissue, and blood stream infections. It is the leading cause of human disease not only in hospitalized individuals but also in individuals living in community and responsible for a variety of diseases ranging from mild skin and soft tissue suppurative (pus-forming) infections, food poisoning to highly serious diseases such as osteomyelitis, endocarditis, and toxic shock syndrome (TSS). The threat of antibiotic resistance in S aureus has risen enormously for several years and the health costs have increased dramatically. Different figures were provided by different nations regarding annual mortality due to antibiotic resistance with 22 000 extra deaths in the United States, 25 000 in Europe, and 12 500 in France.1 Mortality due to methicillin-resistant Staphylococcus aureus (MRSA) is the leading cause of mortality due to bacterial infections, and the number of serious infections due to resistant strains has decreased in recent years. The pathogenesis of staphylococcal infections is multifactorial. However, there is some correlation to the presence of certain virulence factors with a particular disease. Therefore, timely detection of these virulence factors is crucial for undertaking appropriate therapeutic interventions. Molecular methods play an important role in detection and differentiation of pathogens. Numerous techniques have been reported for detection of S aureus virulence factors such as antibody,2–5 polymerase chain reaction (PCR),6,7 real-time PCRs (RT-PCRs),8 and aptamer-based9–11 methods. Many other sensitive methods such as immuno-PCRs,12 mass spectrometric analysis,13 and biosensor techniques14,15 are also reported.
Despite its high incidence and frequency of causing lifethreatening and drug-resistant infections, there is no successful vaccine to prevent S aureus infections. The initial efforts to develop a staphylococcal vaccine that targeted the capsular polysaccharides similar in line with other bacterial pathogens have not been met with success. However, vaccine therapies still hold great promise in broadening the available clinical tools against the global menace of antibiotic-resistant S aureus infections. Antibodies directed against the virulence determinants could neutralize these components and hence may help in reducing the severity of infection. Because toxins are prominent virulence determinants, targeting them and providing the antibodies as passive therapy might render the infections less invasive. The antigens which could induce both humoral and cell-mediated memory immune responses that might prevent the recurring infections elaborated. In this review, an updated information about S aureus virulence factors, pathogenesis, clinical burden, recent advances in S aureus diagnostics, therapy, and prophylaxis.
S aureus General Features, Growth, and Metabolism
Staphylococcus aureus is a gram-positive organism with aerobic to facultative anaerobic lifestyle and colonizes skin, nares, and axillae of humans. Staphylococcus aureus is a catalase-, urease-, and phosphatase-positive organism with most strains secreting coagulase and it also ferments mannitol sugar to lactic acid. Testing for catalase is an important criterion to distinguish Staphylococci from Streptococci and coagulase test for distinguishing S aureus from S epidermidis. It reduces nitrates to nitrites, liquefies gelatin, and is methyl red and Voges-Proskauer test positive. Staphylococcus aureus is lipolytic (lecithinase) when grown on media containing egg yolk. Staphylococcus aureus reduces tellurite in media containing potassium tellurite and produces shiny black color colonies. All strains of S aureus produce a heat-stable thermonuclease which has both endonuclease and exonuclease properties and can degrade both RNA and DNA. Staphylococcus aureus grows in irregular clusters because the cells divide successively in 3 perpendicular planes and the attachment of sister cells may not be in divisional plane but may adjust position while being attached.16 It can remain viable even after many months of air-drying and resists the effect of chemicals and disinfectants.17 Nutritional requirements of S aureus can be met by routine laboratory media, and most strains are metabolically versatile; that is, they can digest proteins, lipids and can ferment a variety of sugars. The average doubling time (mean generation time) of S aureus is as short as 20 minutes.
S aureus and Host Interactions
Staphylococcus aureus is part of normal microflora of humans and is found inhabiting in most human environments. The nares are the primary ecological niche for S aureus; however, multiple sites in the body such as skin, perineum, axillae, vagina, and gastrointestinal tract also were found to harbor this bacterium.18 Staphylococcus aureus in general have a benign or commensal relationship with its host. However, they revert to pathogenic lifestyle once they gain entry into host tissues by injuries, inoculation by syringes, or by direct implantation with medical devices. A successful infection results when there is a shift of balance between host defenses and pathogen virulence mechanisms in favor of the pathogen.
Skin is a major physical and immunologic barrier; the keratinocytes in the epidermis express pattern recognition receptors (PRRs) such as toll-like receptors that recognize pathogen-associated molecular patterns of microbes.19 After recognition, PRRs trigger early cutaneous immune responses such as recruitment of immune cells from circulation to site of recognition. Skin also harbors numerous resident immune cells such as Langerhans cells in the epidermis and dendritic cells, macrophages, mast cells, T and B cells, plasma cells, and natural killer (NK) cells in the dermis. Low temperature and pH of the skin surface resists growth of S aureus. Normal commensal organisms of skin such as S epidermidis and Propionibacterium acnes also prevent colonization and invasion by S aureus by secreting antimicrobial peptides such as phenol-soluble modulins (PSM-α and PSM-δ).20 In addition, keratinocytes in the corneal layer of skin produces antimicrobial peptides that have bacteriostatic and bactericidal properties such as human β-defensins (hBD2, hBD3), cathelicidin (LL-37), and ribonuclease 7.21–23
Colonization of S aureus is mediated by its adherence to surface components such as fibrinogen, fibronectin, and cytokeratins of nasal epithelium or cutaneous keratinocytes. It uses microbial surface components recognizing adhesive matrix molecules for binding such as fibronectin-binding proteins (FnbpA and FnbpB), fibrinogen-binding proteins (ClfA and ClfB), iron-regulated surface determinant (IsdA), and wall teichoic acid (WTA).24,25 Superantigens such as staphylococcal enterotoxin A (SEA), staphylococcal enterotoxin B (SEB), and TSS toxin 1 (TSST-1) enhance fibronectin-mediated and fibrinogen-mediated S aureus colonization during atopic dermatitis by altering the levels of Th2 cytokine profiles (interleukin 4).26 It has additional mechanisms to evade host antimicrobial peptides. For example, iron-regulated surface determinant (IsdA) renders S aureus resistant to β-defensins and cathelicidin and aureolysin, an extracellular metalloproteinase that inhibits cathelicidin activity.27
Staphylococcus aureus has several mechanisms to evade and kill host immune cells and inhibit neutrophil recruitment and antimicrobial activity. Toxins such as α-hemolysin, Panton-Valentine leukocidin (PVL), γ-hemolysin, leukocidin E/D, and PSM lyse the host cells, thus contributing to enhanced virulence. It inhibits the neutrophil recruitment by secretion of chemotaxis inhibitory protein of staphylococci (CHIPS) which reduces the endothelial expression of intercellular adhesion molecule 1 (ICAM-1).28 Neutrophil killing of S aureus by reactive oxygen species is overcome by factors such as S aureus golden pigment29 and superoxide dismutase.30
The success of S aureus strains is due to a unique combination of genetic factors that enable the bacteria to evade host immune system.31 Recent findings suggest that cytolytic PSM-α, cytolysin α-toxin, and the global virulence regulator (agr) have demonstrated important roles in experimental skin infection models.32 It was reported that high WTA amounts might permit S aureus to amplify early responses to abscess formation, thereby creating a microenvironment that protects bacteria from host responses.31 Abscess formation and colony forming unit increase was observed when purified WTA along with bacterial inoculum of WTAlow producers was injected. Wall teichoic acid synthesis is one of the mechanisms that certain MRSA use to gain virulence and therefore could be an ideal target for development of novel anti-infective strategies. Therefore, understanding the host-pathogen interactions is important to identify targets for drug design, designing novel vaccines, and antibody-based therapies.
Clinical Significance of S aureus
Staphylococcus aureus has been a major human pathogen throughout the history and is also the leading cause of bacterial infections worldwide. It is responsible from mild to life-threatening diseases and can potentially infect any tissue in the human body. Among the various S aureus infections, they can be broadly classified into (1) superficial skin and soft tissue infections (SSTIs); (2) systemic and life-threatening infections such as endocarditis, osteomyelitis, pneumonia, meningitis, and bacteremia; and (3) toxinoses such as food poisoning, scalded skin syndrome, and TSS.33 Severity of infection in general is dependent on virulence of the particular strain, inoculum size, and immune status of the individual. Staphylococcal infections are typically characterized by abscess filled with pus and damaged leukocytes surrounded by necrotic tissue. Staphylococcus aureus infections are caused either by autoinfection, infection with own carrier strain, or by cross infection, infection due to strain transmitted from another individual. Staphylococcus aureus has gained resistance to every antimicrobial therapy introduced so far. The massive consumption of antibiotics over the past 50 years has led to the rise in antibiotic resistance, and by far, the resistance against the methicillin has gained utmost significance due to rising public health burden and mortality in comparison with methicillin-susceptible strains.34 Although S aureus is an opportunistic pathogen, there are certain risk factors that increase the likelihood of an infection. Ideally, opportunistic pathogens attack when the body defenses are weakened.35 Skin breakage and or immunosuppression along with nasal carriage are the major risk factors for S aureus infections.36 Nasal carriage varies between individuals and is one of the major risk factors for subsequent S aureus infections.37 In a general population, the average carrier rate is 37% (19%–55%) with some subpopulations showing a higher percentage such as patients with diabetes mellitus, human immunodeficiency virus, dialysis patients, and patients with atopic dermatitis.37,38 Being a carrier is an important predisposition to subsequent infections. Staphylococcus aureus is the cause of large percentage of blood stream (22%) and SSTIs (39%).39 Methicillin-resistant S aureus which was previously restricted to hospitals is increasingly seen in community. Worldwide, community-associated MRSA (CA-MRSA) is one of the major causes of SSTIs and sepsis cases. Two CA-MRSA clones USA300 and USA400 account for 60% to 75% of all S aureus infections in the community.40
S aureus Pathogenesis
Staphylococcus aureus is the most common cause of SSTIs, endocarditis, and second frequent cause of bacteremia. It is also a predominant cause of nosocomial-acquired infections such as intravenous catheter-associated infections, ventilator-associated pneumonia, postsurgical wound infections, invasive infections in neutropenic patients, and in patients undergoing solid organ or hematopoietic cell transplantations.41 Methicillin-resistant S aureus kills ~19 000 hospitalized patients annually in United States, which is similar to combined deaths caused by AIDS, tuberculosis, and viral hepatitis.42 Staphylococcus aureus can evade host defenses and antimicrobials by growing and persisting on biofilms formed on surfaces of the hosts and prosthetic devices.43 Staphylococcus aureus bacteremia (SAB) may be complicated by endocarditis, metastatic infections, or sepsis.44 Endothelial cell is central to all pathogenic process and its activation leads to endovascular infections. Staphylococcus aureus binds by adhesin-receptor interactions and is phagocytized inside the endothelial cells. Intracellular environment protects S aureus from host defense mechanisms and antibiotics. It was also reported that intracellular milieu of endothelial cell favors formation of small colony variants (SCVs).45 These factors contribute to recurrent and persistent infections. Staphylococcus aureus escapes host defenses by invading and surviving inside the endothelial cells in patients with endocarditis.46 Staphylococcus aureus also escapes from host defenses by forming SCVs which survive inside host cells without causing damage, and they are capable of reverting to virulent forms resulting in recurrent infection.47,48
Superantigens cause life-threatening TSS that is characterized by rapid onset of high fever, shock, and multiorgan failure. Superantigens are potent T-cell mitogens that bypass the normal antigen presentation and bind directly to invariant regions of major histocompatibility complex class II molecules of antigen-presenting cells. Major histocompatibility complex–bound superantigen attaches to the variable region of β chain receptor of T cells and causes massive expansion of clonal T cells (5%–10% in contrast to 0.01% for a normal processed antigen) leading to massive release of cytokines and chemokines by macrophages and T cells. The cytokines mediate the TSS leading to tissue damage.49 Toxic shock syndrome toxin 1 contributes to 90% cases related to menstruation and is associated with the use of absorbent tampons. Other enterotoxins contribute to 50% of TSS cases that are not related to menstruation.50
Increase in percentage of SAB is due to increase in catheterization.51 Patients with fever after 72 hours of catheter removal have increased risk of complications.52 Incidence of endocarditis is more in case of intravenous drug users, elderly patients, patients with prosthetic values, and hospitalized patients.44 Staphylococcus aureus has a tendency to spread to other sites in the body such as bones, joints, kidneys, and lungs leading to metastatic infections.53,54 Pus collection at these sites serves as potential foci for persistent and recurrent infections.53 Factors such as advance age, immunosuppression, chemotherapy, and invasive procedures may aid in progress of bacteremia and local infections to sepsis. Staphylococcal sepsis presents with fever, hypotension, tachycardia, and tachypnea. Severe cases progresses to multiorgan dysfunction and death.55
Antibiotic Resistance Mechanisms
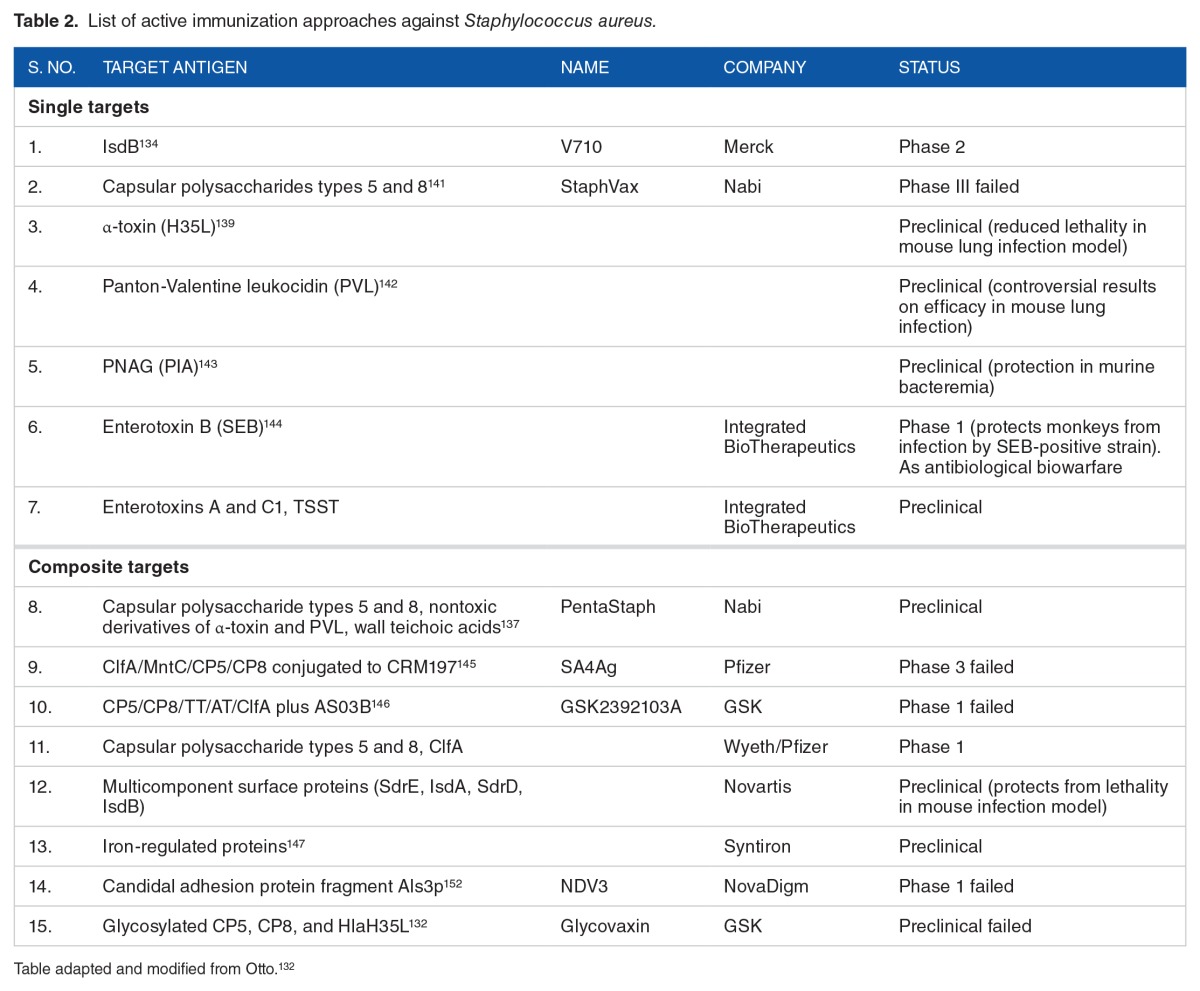
Antibiotic resistance is the resistance of an organism, usually a pathogen to an antimicrobial drug that was effective originally for treating infections. Antibiotic resistance is a serious, ever-growing phenomenon and has emerged as a prominent global health concern in 21st century (http://en.wikipedia.org/wiki/Antibiotic_resistance). Evolution of antibiotic-resistant strains is a natural phenomenon that occurs due to erroneous replication or due to exchange of resistant traits between them. Use and misuse of antibiotics also lead to selection of antibiotic-resistant strains. Multidrug resistance is a common phenomenon among many pathogens such as pneumonia, micrococci, and staphylococci. Staphylococcus aureus is of major concern due to the intrinsic virulence, its ability to cause diverse life-threatening infections, and its ability to adapt to varied environmental conditions. Staphylococcus aureus isolates from blood cultures all over the world are increasingly resistant to multiple antibiotics.56 Mechanisms leading to resistance to some of the broad antibiotic classes are in the following sections and in Table 2.
Table 2.
List of active immunization approaches against Staphylococcus aureus.
Penicillin resistance
Penicillin was discovered in 1928 by Alexander Fleming and is lethal to all sensitive cells by deactivation of cell wall–associated penicillin-binding protein (PBP) transpeptidases. Inactivated transpeptidases are key to cross-linking of peptidoglycan stands which lead to weakened cell wall and death by osmotic lysis.36 Penicillin treatment dramatically improved the prognosis of patients with S aureus infections. However, penicillin-resistant strains were discovered as early as 1942 in both hospitals and community,57 and the incidence was ~80% by 1960 in hospitals and community. This pattern of resistance first appearing in hospitals and later spreading to community is now a common phenomenon observed with each new wave of antibiotic resistance. Resistance to penicillin is mediated by β-lactamase (blaZ), an extracellular enzyme which inactivates the β-lactam nucleus. blaZ gene is located on a transposable element on a plasmid with additional antibiotic-resistant genes (gentamicin and erythromycin). Spread of penicillin resistance occurs through spread of resistant strains.56
Methicillin resistance
To combat penicillin-resistant S aureus, a modified semisynthetic penicillin known as methicillin or meticillin was introduced which is immune to activity of β-lactamase. Soon after its introduction, reports of treatment failure with methicillin occurred by evolution of MRSA.58 Methicillin resistance is mediated by chromosomally located mecA gene which codes for an altered PBP called PBP2a.59 The mecA gene is part of a mobile genetic element (MGE) known as staphylococcal cassette chromosome mec (SSCmec).60 PBP2a substitutes for other PBP in cross-linking of peptidoglycan chains because of its low affinity to β-lactams and therefore enables staphylococci survival even in high concentrations of these agents. The resistance to methicillin confers resistance to other β-lactams such as cephalosporins.61 The therapeutic outcome of infection from an MRSA strain is more severe than from a methicillin-sensitive S aureus (MSSA) strain not only due to enhanced virulence but also due to the fact that MRSA occurs in older hospitalized patients and also due to limited antimicrobial drugs available to treat MRSA. Similar to penicillin resistance, MRSA strains carry multiple antibiotic-resistant genes. Methicillin-resistant S aureus has progressed into an important pathogen of humans and is endemic in hospitals worldwide. Recently, it has emerged in community with increased severity as CA-MRSA. The high mortality associated with some of the CA-MRSA infections is of particular concern. High morbidity of infections associated with CA-MRSA may be due to the presence of enterotoxins and PVL toxins. Treatment of MRSA-associated infections has become complicated owing to remarkable ability of this organism to develop antibiotic resistance.
Quinolone resistance
Fluoroquinolones were first introduced to treat gram-negative bacterial infections in 1980s. Due to broad antibacterial spectrum against gram positives, they have been used to treat infection caused by pneumococci and staphylococci.56 Quinolone resistance quickly emerged in strains with methicillin resistance due to high antibiotic selection pressure in hospital setting resulting in selection and spread of resistant strains. Fluoroquinolone resistance is due to spontaneous chromosomal mutations in antibiotic targets, topoisomerase IV, or DNA gyrase or by the induction of multidrug efflux pump.62 When quinolones are used to treat infections caused by other bacterial pathogens, the resident S aureus strains are likely to get exposed to suboptimal concentrations and are therefore at risk of colonization with resistant strains. Resistance to quinolones is achieved by stepwise acquisition of chromosomal mutations. ParC subunit mutations of topoisomerase IV are more critical for quinolone resistance in staphylococci as they are the primary drug targets.63 Recently, there have been reports of plasmid-mediated resistance mechanisms, including the quinolone resistance proteins such Qnr, Aac(6′) Ib-cr, and QepA.64
Vancomycin resistance
Increased use of vancomycin to treat bacterial infections caused by MRSA, Clostridium difficile, and enterococci paid the way for emergence of vancomycin-resistant S aureus (VRSA). The first report of vancomycin-intermediate S aureus (VISA; minimum inhibitory concentration [MIC]: 8–16 µg/mL) came from Japan followed by more cases from many nations.65,66 It was followed by reports of appearance of vancomycin-resistant strains with total resistance (MIC: >128 µg/mL) and a different mechanism of dissemination. In VISA strains, resistance is mediated by chromosomally located vanA; in contrast, VRSA acquire vanA operon by conjugal transfer from Enterococcus faecalis which is a more efficient means of disseminating resistance genes. Resistance is conferred by increased cell wall biosynthesis which leads to abnormally thick walls. The thick peptidoglycan wall ensnares the vancomycin within cell wall, denying the access to its cytoplasmic target N-acetyl-muramic acid precursor.67,68
Antibiotics have been considered as innovative therapy for many decades. In most cases, after widespread dissemination and prescription, they have been abandoned when it is not economically viable or it is not essential to pharmacopoeia. However, one interesting observation was that only 12.8% of invasive isolates were resistant to methicillin in some hospitals in 2015.69 Some workers have even reported strains that are susceptible to penicillin. (Chabot et al, 2015).70,71 It is essential that we maintain the full repertoire of all antibiotics as part “revival of old antibiotics” to face a particular therapeutic situation. Interestingly, C difficile infections have become more common hospital-associated infections than MRSA infections which have decreased dramatically.72 Some argue that the exaggeration which presently exists regarding antimicrobial resistance is likely an evolutionary trend of our societies to panic to when faced with new phenomenon (Duborg et al, 2015).73
Clinical burden due to S aureus infections
Health care systems of many areas in the world including North America, Europe, Australia, and Asia have witnessed increasing levels of MRSA due to epidemics of highly transmissible clones. However, the true extent of MRSA is not known correctly. In many countries, surveillance is mandatory only in severe forms of disease such as bacteremia. It is highly possible that the percentage of population presenting with actual disease is only the “tip of the iceberg” and that the actual clinical spectrum includes all possible individuals colonized with MRSA but may never develop any clinical disease but can be dangerous to others (Gould, 2005).74 One important factor often forgotten is the additional economic burden incurred on the patients and health care systems. With the increasing incidences of MSSA and MRSA infections, there is a gradual increase in rates of bacteremia and huge additional costs toward treatment. Added to this failure of treatment due to inappropriate antimicrobials or lack of efficacy of anti-MRSA drugs, excess toxicity of new antimicrobials over routine ones is likely to increase the morbidity and mortality. Financial burden of MRSA is very high given the wide spectrum of clinical infections. Direct costs include providing care to MRSA-infected patients, antibiotic treatment costs, indirect costs such as morbidity and diminished quality of life, and infrastructure costs of surveillance and control (Gould, 2005). In one study, after reviewing subjects, extra costs for treatment was estimated in the range of US $3000 to US $30 000 depending on clinical infection and severity.75
Different classes of S aureus virulence factors
Staphylococcus aureus produces many potential virulence factors belonging to various classes categorized based on their functionality such as adherence, invasion and penetration, host evasion, enzymes, toxins, and surface proteins. Some of the major classes of virulence factors that contribute to S aureus infection capabilities include (1) surface proteins (adhesins, clumping factors, IsdA, fibrinogen-binding, and fibronectin-binding proteins) that are involved in the adherence and colonization of host tissues; (2) invasins that promote bacterial spread in host tissues (leukocidins, kinases, and hyaluronidase); (3) surface factors which inhibit phagocytic engulfment (capsule); (4) biochemical properties that enhance their survival abilities inside the host (carotenoids and catalase); (5) immunological disguises (coagulase and protein A); (6) membrane-damaging toxins that lyse host cells (hemolysins, leukotoxin, and leukocidin); (7) secretory toxins that damage host tissues and promote symptoms of disease (enterotoxins A-G, TSST-1, exfoliative toxin); (8) inherent and acquired resistance to antimicrobial agents.16 α- and γ-hemolysins are encoded in the core genome and thus are produced by most strains. Toxins such as enterotoxin A, exfoliative toxins, TSST-1, and PVL are encoded on MGEs of bacteriophages and hence are present in only certain strains.76 Staphylococcus aureus is capable of sensing the surrounding environment and adjust the production of virulence factors suitable for colonization, dissemination, and for causing infection.77 Successful infection of a strain into specific host is multifactorial and depends on the virulence factors secreted by the strain. However, there are certain correlations to the expression of particular virulence determinants which suggest their involvement in certain diseases. Expression of secretory toxins occurs primarily during postexponential growth phase and is controlled by at least 3 global regulatory systems, namely, the accessory gene regulator (agr), the staphylococcal accessory regulator (sar), and extracellular protein regulator (xpr).78 Evidence for staphylococcal matrix-binding proteins as virulence factors came from adherence assay studies involving defective mutants. Defective fibrinogen-binding and fibronectin-binding S aureus mutants have reduced virulence in rat endocarditis model.79 Mutants deficient in collagen-binding protein has reduced virulence in mouse septic arthritis model.80 The role of some of the important classes of virulence factors such as hemolysins, leukocidins, and superantigens needs more discussion.
S aureus enzymes
The primary role of staphylococcal enzymes is to provide the nutrients for cell growth and division, and only certain enzymes play key role in the pathogenesis. Proteolytic enzymes of S aureus are involved in the inactivation of antimicrobial peptides and also for modulating and activating other virulence factors (zymogens) such as clumping factors, staphylococcal protein A (SpA), and fibrinogen-binding proteins. The major proteolytic enzymes consist of a metalloproteinase (aureolysin, Aur), a serine glutamyl endopeptidase (serine protease, SspA), and 2 related cysteine proteinases referred to as staphopain (ScpA) and the cysteine protease (SspB).81 Hyaluronidase produced by most S aureus strains helps in degrading hyaluronic acid from connective tissue and promotes bacterial spread inside host tissues. Coagulase protects bacteria from host defenses by forming fibrin clot around the foci of infection.82
Hemolysins
Among the membrane-damaging toxins, α-hemolysin is the most potent pore-forming toxin, expressed as monomer by almost all the clinical isolates of S aureus. α-Hemolysin monomers oligomerize to form a functional heptameric toxin with a central pore through which cell contents are leaked. There is a direct correlation between the levels of α-hemolysin expression and the virulence of a particular strain suggesting its prominent role in pathogenesis.83 Platelets and monocytes are the most susceptible cells to the action of α-hemolysin, and the method of cells lysis is likely by osmotic lysis.16 β-toxin is a sphingomyelinase which damages membranes rich in lipids, and most of the human isolates do not express this toxin. It is encoded by a lysogenic bacteriophage.84
Leukocidins
The PVL and γ-hemolysins are the staphylococcal bicomponent toxins with leukocytotoxic activity requiring the action of 2 components, the S and the F subunits. Leukocidins are associated with a total of 5 genes: γ-hemolysins are encoded by 3 ORFs, hlgA, hlgB, and hlgC, and PVL is encoded by 2 cotranscribed ORFs, the lukS-PV and lukF-PV. Among these, hlgA, hlgC, and lukS-PV function as S component, whereas hlgB and lukF-PV function as F component. The γ-hemolysin is present in almost 99% of S aureus strains, and hence, its involvement in pathogenesis is difficult to ascertain. In contrast, PVL toxin has increasingly been associated not only with community-acquired primary SSTIs but also with severe necrotizing pneumonia in young and healthy individuals.85
Phenol-soluble modulins
Phenol-soluble modulins are recently discovered amphipathic, α-helical peptides secreted by members of staphylococci. Phenol-soluble modulins are key virulence determinants in highly virulent S aureus strains. Phenol-soluble modulin α peptides of S aureus lyse neutrophils after they are phagocytized. Phenol-soluble modulins are also key factors for biofilm formation and their dissemination in biofilm-associated infections. The surfactant properties of PSMs facilitate their growth on epithelial surfaces. Phenol-soluble modulin can be grouped in to smaller (~20–25 amino acids [aa]) α-type PSMs and longer (~44 aa) β-type PSMs.86
Superantigens
Toxic shock syndrome is a rare condition associated with menstruating women using tampons and is characterized by rapid onset of fever and multiorgan failure. This condition is caused by TSST-1 belonging to a class of staphylococcal superantigens which causes massive activation of T lymphocytes.87 Gene encoding TSST-1 is located on a less transmissible pathogenic island designated as SapI 1, and hence, tst-1 is present in only few restricted clones.88 Staphylococcal enterotoxins (SEs) belong to a group of structurally related superantigen family of toxins whose presence is correlated with increased virulence in nosocomial infections. Staphylococcal enterotoxin A has been associated with more severe infections such as staphylococcal food poisoning (SFP) and septic shock in comparison with other enterotoxins. Most of the enterotoxins are carried by plasmids, phages, pathogenicity islands, or MGEs.89 Exfoliative toxins (ETA, ETB, ETC, and ETD) cause exfoliation of skin epidermis followed by secondary infections. ETA and ETB are the important isoforms in humans predominantly affecting neonates and are associated with staphylococcal bullous impetigo and staphylococcal scalded skin syndrome. Prevalence of exfoliative toxins is not so frequent in S aureus.87
Staphylococcal food poisoning
Staphylococcus aureus is one of the most frequent pathogens responsible for food-borne outbreaks worldwide. It causes SFP after ingestion of foods containing preformed heat-stable enterotoxins. Contamination in SFP cases occurs commonly due to improper or extensive manual handling of foods rich in proteins combined with inadequate heating and improper storage.91 Foods commonly contaminated with SEs are meat and meat products, poultry and egg products, milk and milk products, and confectionary products such cream-filled pastries and cakes.92 Staphylococcal food poisoning was the fourth most frequent cause of food-borne illness in European Union in 2008 (EFSA, 2010).89 Although S aureus cells can be killed by heating, the enterotoxins are very stable even after rigorous heating. Staphylococcal enterotoxins are resistant to proteases such as pepsin, trypsin, papain, and rennin and thus they are active even after ingestion in the intestine. At present, there are 23 enterotoxins or enterotoxin-like genes.93 Staphylococcal enterotoxins are globular, single-polypeptide proteins which are related structurally with molecular weights ranging from 22 to 29 kDa. Staphylococcal food poisoning is associated with rapid onset of symptoms within 2 to 8 hours from the time of ingestion of contaminated food. Symptoms typically include nausea, vomiting, abdominal cramping, and occasionally with diarrhea and fever.94 Severity of SFP is dependent on amount of SE ingested and the health status of the individual. In most cases, the symptoms subside within 24 to 48 hours; however, in case of infants and elderly people, it requires hospitalization.95 In cases of severe dehydration, it requires supplementation with intravenous fluid administration. Staphylococcal enterotoxin A is the most frequently encountered SE among SFP cases.96 Enterotoxin A (sea) is very different from all other SE genes such as enterotoxin B (seb), enterotoxin C (sec), and enterotoxin D (sed) because it is carried by polymorphic family of lysogenic or temperate phages.97
Detection methods for S aureus and its toxins
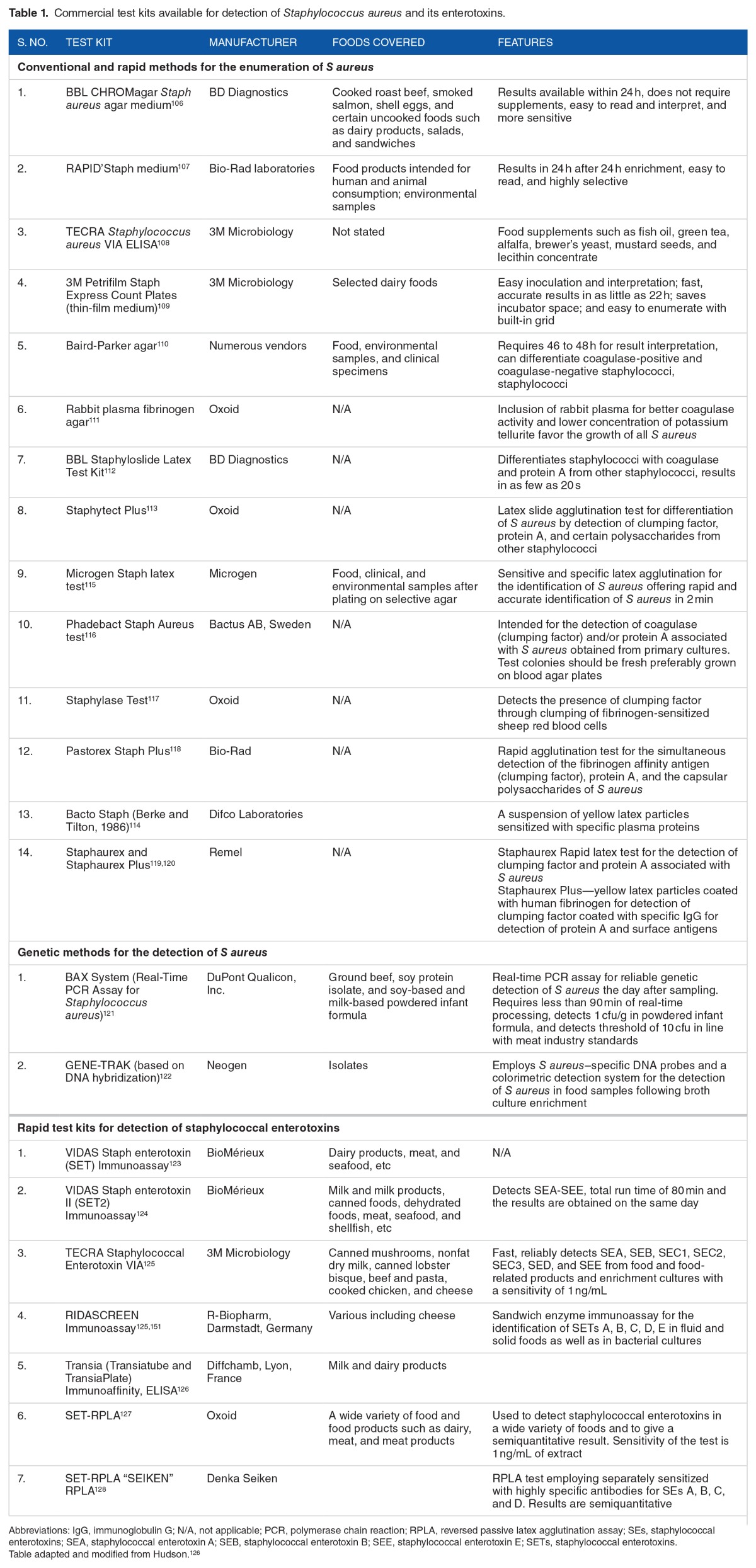
Pathogenesis of S aureus diseases is a multifactorial phenomenon. However, there is some relationship with the presence of certain virulence factors to a particular disease. Although S aureus produces various toxins and enzymes, there is direct correlation between virulence of a particular strain with the amount of α-hemolysin secreted. The presence of this bacterium or its enterotoxins in processed foods is a general indication of poor sanitation. Mere isolation of S aureus–viable cells may not be sufficient to cause food poisoning. It should have the capacity to secrete enterotoxins (SEs). Staphylococcal enterotoxins also play an important role in food poisoning and TSS cases. Staphylococcal enterotoxins are globular, single polypeptides which constitute a family of related proteins with similarities at structural and aa levels. Although heat treatment used commonly in food processing industries destroys S aureus vegetative cells, the heat-stable enterotoxins secreted by this organism are resistant to high temperatures for extended periods. Food intoxication caused by SEs is referred to as SFP and is one of the common forms of foodborne illnesses reported worldwide. Staphylococcal food poisoning is characterized by nausea, vomiting, and abdominal cramps. Staphylococcal enterotoxins are also responsible for autoimmune responses due to their superantigenic nature resulting in TSS. Staphylococcal enterotoxin A is one of the most commonly encountered enterotoxins among SFP cases.96 Staphylococcal enterotoxin B is another enterotoxin responsible for food poisoning and is also a potent T-cell mitogen and hence is listed as a category B bioweapon agent.98 Concentrations of 0.5 to 1 ng/mL of SEs are sufficient to induce food poisoning. Therefore, detection and quantification of SEs from food is a more appropriate approach than detection of S aureus viable cells from foods. Laboratory methods for identification of S aureus from food sample, wound, or blood culture require isolation and biochemical test procedures which require considerable time and resources. Several methods have been reported for rapid identification so that most appropriate therapeutic interventions can be undertaken. Commercially, many kits are available for the enumeration of S aureus from food and environmental samples and also for detection of SEs from isolates as well as food samples. Polymerase chain reaction has revolutionized several areas of molecular biology particularly in the field of molecular diagnostics of infectious diseases. Polymerase chain reaction methods for S aureus identification includes PCRs for species-specific genes such as 16S RNA,99 thermonuclease (nuc),100 and acriflavine resistance101 gene. Molecular methods play an important role in detection and differentiation of pathogens. Numerous techniques have been reported for detection of SEs such as antibody,4,5 PCR,6,7 RT-PCRs,8 and aptamer-based9–11 methods. Many other sensitive methods such as immuno-PCRs,12 mass spectrometric analysis,13 and biosensor techniques14,15 are also reported. Although these methods are sensitive, they are relatively expensive and thus cannot be used in routine testing of multiple samples.
Among the immunological assays, Western blots, radio immunoassay, enzyme-linked immunosorbent assays (ELISAs), and reversed passive latex agglutination assay have been described for detection and quantification of exotoxins such as α-hemolysin, enterotoxins, and PVL toxins. Immunoassays could be used to detect SEs directly from culture or from contaminated food material. There are many commercially available kits such as VIDAS, TRANSIA, TECRA, and RIDASCREEN for the detection of SEs available commonly in sandwich ELISA formats (Table 1). Many in-house assays4,5,96 have also been reported for detection of SEs. Among the various antibody-based formats reported so far, immuno-PCR is a sensitive diagnostic technique which combines the specificity of ELISA with the sensitivity of PCR and it offers the advantages of high sensitivity and easy automation for detection multiple analytes by differential capture of antigens. Immuno-PCR has established itself as a potential diagnostic tool and has been applied for the detection of various bacterial102 and viral pathogens,103 bacterial toxins,104 and mycotoxins.105 Most of the immunoassays employ antibodies from mammalian sources such as rabbit, mice, sheep, and goat. The major hindrance with the specificity of these immunoassays is the presence of a 42-kDa SpA secreted by all S aureus strains. Staphylococcal protein A is an immunoglobulin-binding protein present on cell wall and is also secreted into the medium during exponential growth phase. Staphylococcal protein A causes false positives in antibody-based tests involving S aureus antigens due to its ability to bind various classes and subclasses of immunoglobulins. Staphylococcal protein A mediates this activity by binding to Fc region of most immunoglobulin classes and to F(ab)2 region of certain immunoglobulin classes. A variety of methods have been proposed to overcome SpA interference in immunoassays. However, there are limitations with these assays and are not completely free from the effect of protein A.
Table 1.
Commercial test kits available for detection of Staphylococcus aureus and its enterotoxins.
In recent times, there is an increasing use of antibodies from avian (immunoglobulin Y [IgY]) sources, especially from chickens, because raising antibodies from chickens are more convenient, hygienic, inexpensive, and isolation does not require invasive methods unlike from mammalian sources. Egg yolks are abundant sources of IgY, and single yolk can yield IgY in the range of 10 to 20 mg/mL. There are several advantages with IgY and, most importantly, IgY does not have any affinity to immunoglobulin-binding proteins such as protein A, protein G, and protein L. Chicken antibodies were used in many assays where there is a marked effect of SpA on immunoassays due to its binding ability to mammalian immunoglobulins.2,3,129
Next-generation sequencing (NGS) offers potential solution to challenges in detection of infectious diseases. Next-generation sequencing offers huge potential in sequencing all the nucleic acids present in a sample allowing limitless multiplex interrogations, thereby providing higher levels of diagnostic interpretation through complete characterization of genomic content.130 However, an unbiased NGS requires a substantial amount of sequence depth to separate low-prevalence pathogens from overwhelming host nucleic acids. Targeted NGS such as using pathogen-specific signatures for amplification could be a possible mitigation strategy. Next-generation sequencing would offer immense aid in diagnosing serious S aureus infections such as bacteremia and pneumonia and especially in low-income countries.
Treatment, therapies, and prevention
Most of the S aureus isolated from hospitals and community are resistant to multiple antibiotics which therefore makes the treatment of S aureus infections complicated. Treatment of infections by multidrug-resistant S aureus is possible only with last line of antibiotics such as vancomycin and linezolid. Additional nonspecific mechanisms such as biofilm formation on medical devices also aid in the resistance to antimicrobial agents. At present, little interest is being shown for the development of novel antibiotics due to the high cost, limited success rate, and possible emergence of antibiotic resistance. Therefore, researchers have intensified their interest toward the development of vaccines and therapeutic antibodies because they can be raised easily and inexpensively in comparison with development of novel antibiotics. Moreover, vaccination might be beneficial to people at high risk such as dialysis patients, patients at risk of endocarditis, patients undergoing surgery, sports persons, prison inmates, and health care workers who are the potential sources of dissemination of hospital-associated MRSA in hospitals and to patients. However, in contrast to other bacterial pathogens, there is no vaccine available yet that stimulates active immunity against staphylococcal infections in humans. This may be due to the fact that S aureus is a permanent or transient colonizer in part of the population and it has developed mechanisms to thwart human immune mechanisms such as immunologic disguises, toxins that lyse white blood cells, avoiding complement deposition, dysregulated immune hyperactivation, and evasion of phagocytic killing.41,131,132 In addition, hyperimmune serum or monoclonal antibodies could be given to patients undergoing surgery as a form of passive immunization. There is evidence that preexisting antibodies against TSST-1 protects people from TSST-1–induced disease.133 Therefore, this research is aimed at increasing the preexisting antibody titers to some of the key virulence determinants to reduce the severity of infection. Several active and passive immunization strategies are being undertaken and are mainly targeted at molecules involved in pathogenesis.
The selection of antigen for Merck V710 vaccine is based on study involving screening S aureus peptide libraries with human serum. The surface protein IsdB which plays role in heme acquisition and iron uptake was selected as antigen. This vaccine was found highly immunogenic and was protective against diverse strains in animal infection models.134,135 Due to promising results with capsular polysaccharides as vaccine targets with Haemophilus influenza and Streptococcus pneumoniae, Nabi has developed a StaphVax vaccine based on type 5 and 8–based capsular polysaccharides bound to pseudomonal exotoxoid A as carrier. Passive immunization studies were promising with mouse and rat infection models of bacteremia. However, in phase 3 clinical trials involving hemodialysis, patient’s protection was seen only until 40 weeks. Decrease in protection was correlated with decrease in S aureus antibodies. Therefore, the company has stopped further development of StaphVax vaccine. However, StaphVax could be administered to patients who need protection for shorter duration or people visiting hospitals for short duration such as surgery. Despite the presence of impressive opsonophagocytic anticapsular antibodies, they failed to protect patients for longer durations. Failure of capsular polysaccharides as vaccine candidates in S aureus in contrast to success in H influenza is due to the fact that role of capsular polysaccharide in S aureus pathogenesis is very limited.136,137
α-Hemolysin is a potent cytolytic toxin encoded on core genome and is present in most S aureus strains which makes it an ideal vaccine target. Earlier studies involving α-toxin and whole killed S aureus did not show efficacy in preventing infection in dialysis patients.138 However, a nontoxic, nonhemolytic variant of α-hemolysin H35L has proven to be valuable for vaccine development.139 Role of PVL as a vaccine candidate is highly controversial as its role in contribution to pathogenesis. Panton-Valentine leukocidin had no protective effect against CA-MRSA strain USA300 clone in mouse lung infection model; however, α-hemolysin showed strong protective effect.140 After failure of StaphVax, Nabi has further added 3 antigens in its vaccine, namely, WTA, nontoxic α-hemolysin variant, and PVL. This vaccine is now called PentaStaph owing to the 5 antigen components in the formulation. Furthermore, a variety of targets such as surface proteins and adhesins have been evaluated as vaccine candidates in different studies. These active immunization strategies have been summarized in Table 2.
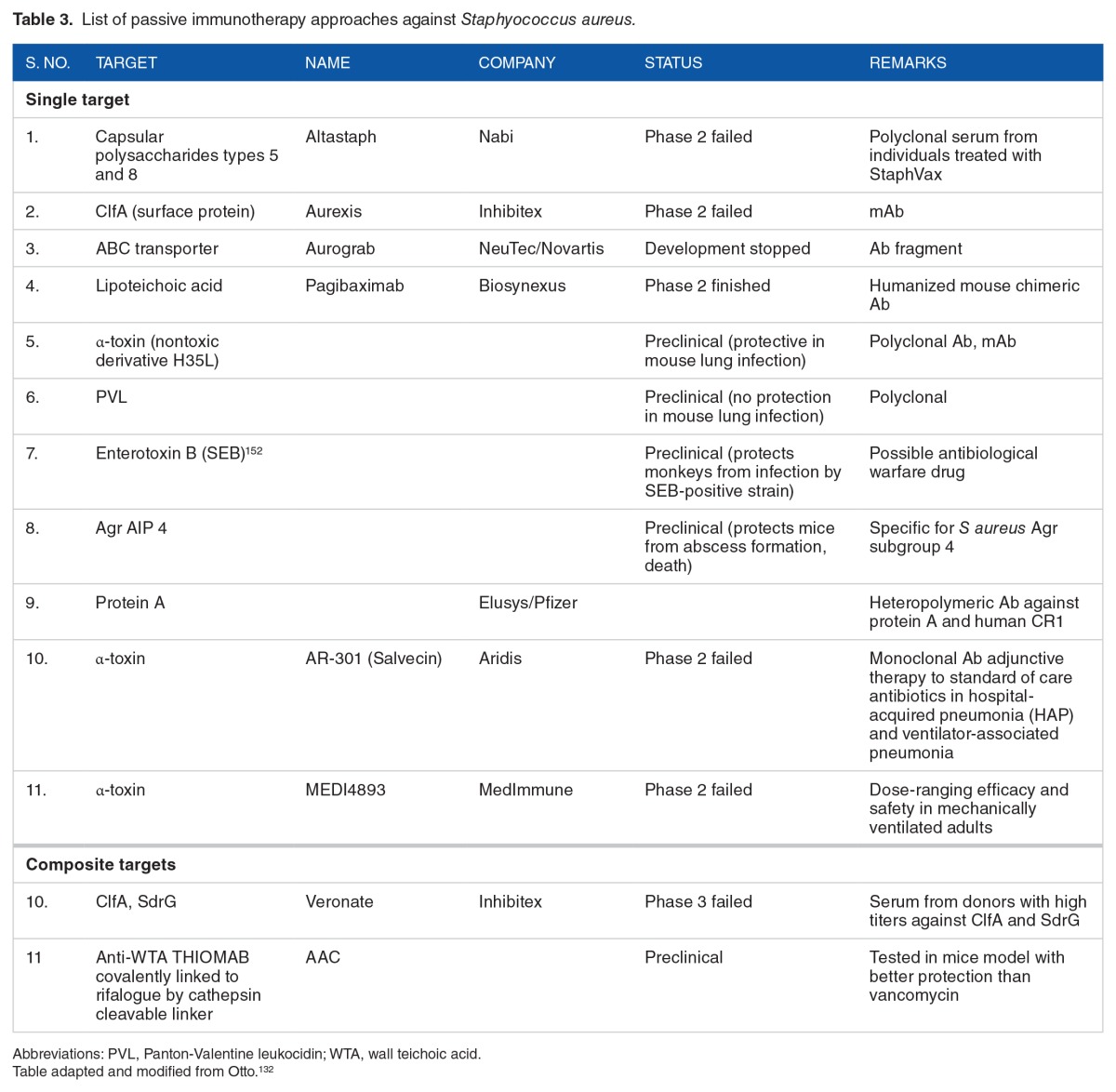
Due to the limited success with vaccines strategies against S aureus, there has been shift toward passive immunotherapy approaches. Most of these strategies are aimed at neutralizing the virulence determinants in particular toxins and surface components (Table 3). Because S aureus has a diverse array of virulence factors, passive immunotherapy approaches should be aimed at several different virulence determinants. A multivalent antigen offers more promise than distinct individual antigens in that they might induce complementary and nonoverlapping immune mechanisms of protection across diverse human populations.
Table 3.
List of passive immunotherapy approaches against Staphyococcus aureus.
It is now understood that the primary immune mechanisms required for protection against S aureus infections include phagocytes and T lymphocytes (Th17 cells).41 In addition, antigen selection should be such that they induce strong humoral as well as T-cell immune responses that react to broadest possible S aureus strains. In this regard, multivalent antigens will be more likely to induce both humoral and T-cell immune responses and might give protection to broad array of S aureus strains. Among T cells, Th17 cells are important in vaccine-mediated protection against S aureus in mouse model and they act by recruitment of neutrophils to the site infection and promoting their killing.148 Invasive infections of S aureus result in generation of memory immune response as seen by high antibody titers postinfection. However, whether this memory immune response will protect against recurrent infection is not well established.149,150 In various studies involving disparate populations, it was observed that 10% to 30% of cutaneous abscesses resulted in recurrence.151,152 Therefore, natural infection with S aureus does not result in a protective immune memory response which leads to further recurrence. Possible reasons for failure with traditional vaccines may be due to immune evasion mechanisms of S aureus, for example, the killing of phagocytes by leukolytic toxins. Lessons from clinical and preclinical research reports suggest to the use of surface proteins and toxins with proven role in pathogenesis as promising targets for vaccine development. The use of therapeutic antibodies represents a novel, adjunctive, or alternative strategy to specifically target toxins with a demonstrated role in S aureus virulence.
Conclusions
Despite numerous efforts in developing a vaccine for combatting S aureus, no vaccine was successful in providing a memory immune response to previous infection. Lessons from clinical and preclinical research reports suggest to the use of surface proteins and toxins with proven role in pathogenesis as promising targets for vaccine development. The use of therapeutic antibodies represents a novel, adjunctive, or alternative strategy to specifically target toxins with a demonstrated role in S aureus virulence. The development of novel antibody-based therapies might offer hope in treatment of severe and invasive infections as an adjunctive to antibiotic treatment. The antibodies should target and neutralize virulence factors, immune evasion molecules, and surface factors to target them for destruction.
Acknowledgments
The authors are thankful to Vignan’s Foundation for Science, Technology and Research University (VFSTRU) management for providing necessary facilities and permissions for writing this article. Some of the materials and opinions presented in the manuscript are taken from the PhD thesis of P.N.R. which was not published or under consideration for publication elsewhere. Necessary permission was sought from the University for the publication of contents of the thesis. Prakash Narayana Reddy is a National postdoctoral fellow funded by Department of Science and Technology, India.
Footnotes
PEER REVIEW: Five peer reviewers contributed to the peer review report. Reviewers’ reports totaled 632 words, excluding any confidential comments to the academic editor.
FUNDING: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Prakash Narayana Reddy is a National postdoctoral fellow funded by Department of Science and Technology, India.
DECLARATION OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions
PNR is involved in literature collection, writing, editing and revising manuscript. KS involved in proof reading and providing critical correction in language and material. VRD is involved in preparing manuscript outline, proof reading and undertaking manuscript revision.
Criteria for Literature Search and Selection
Data for this review were identified from searches in PubMed, Google Scholar, and ScienceDirect and from references of popular articles. Some of the data presented were also identified from the extensive literature collections of the authors. Some of the key words for searching and selection of literature were S aureus, virulence factors, antibiotic resistance, host-pathogen interactions, superantigens, toxins, detection, immunodiagnostics, ELISA, subunit vaccine, prophylaxis, therapy, etc. Only articles written in English language were chosen for review. No data restriction was set during literature search.
REFERENCES
- 1.Dubourg G, Abat C, Raoult D. Why new antibiotics are not obviously useful now [published online ahead of print January 16, 2017] Int J Antimicrob Ag. doi: 10.1016/j.ijantimicag.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 2.Reddy PN, Shekar A, Kingston JJ, Sripathy HM, Batra HV. Evaluation of IgY capture ELISA for sensitive detection of alpha hemolysin of Staphylococcus aureus without staphylococcal protein A interference. J Immunol Methods. 2013;391:31–38. doi: 10.1016/j.jim.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Reddy PN, Ramlal S, Sripathy HM, Batra HV. Development and evaluation of IgY immunocapture ELISA for detection of Staphylococcus aureus enterotoxin A devoid of protein A interference. J Immunol Methods. 2014;408:114–122. doi: 10.1016/j.jim.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 4.Kuang H, Wang W, Xu L, et al. Monoclonal antibody-based sandwich ELISA for the detection of staphylococcal enterotoxin A. Int J Environ Res Public Health. 2013;10:1598–1608. doi: 10.3390/ijerph10041598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiao DJ, Wey JJ, Tsui PY, Lin FG, Shyu RH. Comparison of LFA with PCR and RPLA in detecting SEB from isolated clinical strains of Staphylococcus aureus and its application in food samples. Food Chem. 2013;141:1789–1795. doi: 10.1016/j.foodchem.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Jeyasekaran G, Raj KT, Shakila RJ, Thangarani AJ, Karthika S, Luzi M. Simultaneous detection of Staphylococcus aureus enterotoxin C-producing strains from clinical and environmental samples by multiplex PCR assay. Ann Microbiol. 2011;61:585–590. [Google Scholar]
- 7.Nagaraj S, Ramlal S, Sripathy MH, Batra HV. Development and evaluation of a novel combinatorial selective enrichment and multiplex PCR technique for molecular detection of major virulence-associated genes of enterotoxigenic Staphylococcus aureus in food samples. J Appl Microbiol. 2013;116:435. doi: 10.1111/jam.12364. [DOI] [PubMed] [Google Scholar]
- 8.Horsmon JR, Cao CJ, Khan AS, Gostomski MV, Valdes JJ, O’Connell KP. Real-time fluorogenic PCR assays for the detection of entA, the gene encoding staphylococcal enterotoxin A. Biotechnol Lett. 2006;28:823–829. doi: 10.1007/s10529-006-9011-0. [DOI] [PubMed] [Google Scholar]
- 9.DeGrasse JA. A single-stranded DNA aptamer that selectively binds to Staphylococcus aureus enterotoxin B. PLoS ONE. 2012;7:e33410. doi: 10.1371/journal.pone.0033410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu A, Zhang Y, Chen W, Wang X, Chen F. Gold nanoparticle-based colorimetric detection of staphylococcal enterotoxin B using ssDNA aptamers. Eur Food Res Technol. 2013;237:323–329. [Google Scholar]
- 11.Huang Y, Chen X, Xia Y, et al. Selection, identification and application of a DNA aptamer against Staphylococcus aureus enterotoxin A. Anal Method. 2014;6:690–697. [Google Scholar]
- 12.Rajkovic A, Moualij BEI, Uyttendaele U, et al. Immunoquantitative real-time PCR for Detection and Quantification of Staphylococcus aureus Enterotoxin B in Foods. Appl Environ Microb. 2006;72:6593–6599. doi: 10.1128/AEM.03068-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sospedra I, Soler C, Manes J, Soriano JM. Rapid whole protein quantitation of staphylococcal enterotoxins A and B by liquid chromatography/mass spectrometry. J Chromatogr A. 2012;1238:54–59. doi: 10.1016/j.chroma.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 14.Sapsford KE, Taitt CR, Loo N, Ligler FS. Biosensor detection of botulinum toxoid A and staphylococcal enterotoxin B in food. Appl Environ Microb. 2005;71:5590–5592. doi: 10.1128/AEM.71.9.5590-5592.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Homola J, Dostalek J, Chen S, Rasooly A, Jiang S, Yee SS. Spectral surface plasmon resonance biosensor for detection of staphylococcal enterotoxin B in milk. Int J Food Microbiol. 2002;75:61–69. doi: 10.1016/s0168-1605(02)00010-7. [DOI] [PubMed] [Google Scholar]
- 16.Todar K. Todar’s online textbook of bacteriology. 2007. http://textbookofbacteriology.net/staph.html. Published.
- 17.Talaro KP, Chess B. Foundations in Microbiology. 8th ed. New York, NY: McGraw-Hill Publishers; 2012. [Google Scholar]
- 18.Sangvik M. Staphylococcus aureus Colonisation and Host-Microbe Interactions [PhD thesis] Tromsø, Norway: University of Tromsø UIT; 2013. [Google Scholar]
- 19.Krishna S, Miller LS. Host-pathogen interactions between the skin and Staphylococcus aureus. Curr Opin Microbiol. 2012;15:28–35. doi: 10.1016/j.mib.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cogen AL, Yamasaki K, Sanchez KM, et al. Selective antimicrobial action is provided by phenol-soluble modulins derived from Staphylococcus epidermidis, a normal resident of the skin. J Invest Dermatol. 2009;130:192–200. doi: 10.1038/jid.2009.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Braff MH, Zaiou M, Fierer J, Nizet V, Gallo RL. Keratinocyte production of cathelicidin provides direct activity against bacterial skin pathogens. Infect Immun. 2005;73:6771–6781. doi: 10.1128/IAI.73.10.6771-6781.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kisich KO, Howell MD, Boguniewicz M, Heizer HR, Watson NU, Leung DY. The constitutive capacity of human keratinocytes to kill Staphylococcus aureus is dependent on β-defensin 3. J Invest Dermatol. 2007;127:2368–2380. doi: 10.1038/sj.jid.5700861. [DOI] [PubMed] [Google Scholar]
- 23.Simanski M, Dressel S, Gläser R, Harder J. RNase 7 protects healthy skin from Staphylococcus aureus colonization. J Invest Dermatol. 2010;130:2836–2838. doi: 10.1038/jid.2010.217. [DOI] [PubMed] [Google Scholar]
- 24.Weidenmaier C, Kokai-Kun JF, Kristian SA, et al. Role of teichoic acids in Staphylococcus aureus nasal colonization, a major risk factor in nosocomial infections. Nat Med. 2004;10:243–245. doi: 10.1038/nm991. [DOI] [PubMed] [Google Scholar]
- 25.Burian M, Rautenberg M, Kohler T, et al. Temporal expression of adhesion factors and activity of global regulators during establishment of Staphylococcus aureus nasal colonization. J Infect Dis. 2010;201:1414–1421. doi: 10.1086/651619. [DOI] [PubMed] [Google Scholar]
- 26.Laouini D, Kawamoto S, Yalcindag A, et al. Epicutaneous sensitization with superantigen induces allergic skin inflammation. J Allergy Clin Immun. 2003;112:981–987. doi: 10.1016/j.jaci.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 27.Sieprawska-Lupa M, Mydel P, Krawczyk K, et al. Degradation of human antimicrobial peptide LL-37 by Staphylococcus aureus-derived proteinases. Antimicrob Agents Ch. 2004;48:4673–4679. doi: 10.1128/AAC.48.12.4673-4679.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Athanasopoulos AN, Economopoulou M, Orlova VV, et al. The extracellular adherence protein (Eap) of Staphylococcus aureus inhibits wound healing by interfering with host defense and repair mechanisms. Blood. 2006;107:2720–2727. doi: 10.1182/blood-2005-08-3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu GY, Essex A, Buchanan JT, et al. Staphylococcus aureus golden pigment impairs neutrophil killing and promotes virulence through its antioxidant activity. J Exp Med. 2005;202:209–215. doi: 10.1084/jem.20050846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karavolos MH, Horsburgh MJ, Ingham E, Foster SJ. Role and regulation of the superoxide dismutases of Staphylococcus aureus. Microbiology. 2003;149:2749–2758. doi: 10.1099/mic.0.26353-0. [DOI] [PubMed] [Google Scholar]
- 31.Wanner S, Schade J, Keinhörster D, et al. Wall teichoic acids mediate increased virulence in Staphylococcus aureus. Nat Microbiol. 2017;2:16257. doi: 10.1038/nmicrobiol.2016.257. [DOI] [PubMed] [Google Scholar]
- 32.Cheung GY, Wang R, Khan BA, Sturdevant DE, Otto M. Role of the accessory gene regulator agr in community-associated methicillin-resistant Staphylococcus aureus pathogenesis. Infect Immun. 2011;79:1927–1935. doi: 10.1128/IAI.00046-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aires de Sousa M, Lencastre H. Bridges from hospitals to the laboratory: genetic portraits of methicillin-resistant Staphylococcus aureus clones. FEMS Immunol Med Mic. 2004;40:101–111. doi: 10.1016/S0928-8244(03)00370-5. [DOI] [PubMed] [Google Scholar]
- 34.Stark L. Staphylococcus aureus: Aspects of Pathogenesis and Epidemiology [Medical thesis] Linköping, Sweden: Linkoping University; 2013. [Google Scholar]
- 35.Davis KA, Stewart JJ, Crouch HK, Florez CE, Hospenthal DR. Methicillin-resistant Staphylococcus aureus (MRSA) nares colonization at hospital admission and its effect on subsequent MRSA infection. Clin Infect Dis. 2004;39:776–782. doi: 10.1086/422997. [DOI] [PubMed] [Google Scholar]
- 36.Stephens AJ. The Development of Rapid Genotyping Methods for Methicillin Resistant Staphylococcus aureus [PhD dissertation] Brisbane, QLD: Queensland University of Technology, Australia; 2008. [Google Scholar]
- 37.Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms and associated risks. Clin Microbiol Rev. 1997;10:505–520. doi: 10.1128/cmr.10.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoeger PH, Lenz W, Boutonnier A, Fournier JM. Staphylococcal skin colonization in children with atopic dermatitis: prevalence, persistence, and transmission of toxigenic and nontoxigenic strains. J Infect Dis. 1992;165:1064–1068. doi: 10.1093/infdis/165.6.1064. [DOI] [PubMed] [Google Scholar]
- 39.Diekema DJ, Pfaller MA, Schmitz FJ, et al. Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997–1999. Clin Infect Dis. 2001;32:S114–S132. doi: 10.1086/320184. [DOI] [PubMed] [Google Scholar]
- 40.Moellering RC. The growing menace of community-acquired methicillin-resistant Staphylococcus aureus. Ann Intern Med. 2006;144:368–370. doi: 10.7326/0003-4819-144-5-200603070-00014. [DOI] [PubMed] [Google Scholar]
- 41.Spellberg B, Daum R. Development of a vaccine against Staphylococcus aureus. Semin Immunopathol. 2012 Mar;34:335–348. doi: 10.1007/s00281-011-0293-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boucher HW, Corey GR. Epidemiology of methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2008;46:S344–S349. doi: 10.1086/533590. [DOI] [PubMed] [Google Scholar]
- 43.Gordon RJ, Lowy FD. Pathogenesis of methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis. 2008;46:S350–S359. doi: 10.1086/533591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lowy FD. Staphylococcus aureus infections. New Engl J Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 45.Vesga O, Groeschel MC, Otten MF, Brar DW, Vann JM, Proctor RA. Staphylococcus aureus small colony variants are induced by the endothelial cell intracellular milieu. J Infect Dis. 1996;173:739–742. doi: 10.1093/infdis/173.3.739. [DOI] [PubMed] [Google Scholar]
- 46.Moreillon P, Que YA, Bayer AS. Pathogenesis of streptococcal and staphylococcal endocarditis. Infect Dis Clin N Am. 2002;16:297–318. doi: 10.1016/s0891-5520(01)00009-5. [DOI] [PubMed] [Google Scholar]
- 47.Kahl B, Herrmann M, Everding AS, et al. Persistent infection with small colony variant strains of Staphylococcus aureus in patients with cystic fibrosis. J Infect Dis. 1998;177:1023–1029. doi: 10.1086/515238. [DOI] [PubMed] [Google Scholar]
- 48.Proctor RA, van Langevelde P, Kristjansson M, Maslow JN, Arbeit RD. Persistent and relapsing infections associated with small-colony variants of Staphylococcus aureus. Clin Infect Dis. 1995;20:95–102. doi: 10.1093/clinids/20.1.95. [DOI] [PubMed] [Google Scholar]
- 49.Marrack P, Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990;248(4956):705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- 50.Bohach GA, Fast DJ, Nelson RD, Schlievert PM. Staphylococcal and streptococcal pyrogenic toxins involved in toxic shock syndrome and related illnesses. Critical Reviews in Microbiology. 1990;17:251–272. doi: 10.3109/10408419009105728. [DOI] [PubMed] [Google Scholar]
- 51.Wergeland HI, Haaheim LR, Natås OB, Wesenberg F, Oeding P. Antibodies to staphylococcal peptidoglycan and its peptide epitopes, teichoic acid, and lipoteichoic acid in sera from blood donors and patients with staphylococcal infections. J Clin Microbiol. 1989;6:1286–1291. doi: 10.1128/jcm.27.6.1286-1291.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Steinberg JP, Clark CC, Hackman BO. Nosocomial and community-acquired Staphylococcus aureus bacteremias from 1980 to 1993: impact of intravascular devices and methicillin resistance. Clin Infect Dis. 1996;23:255–259. doi: 10.1093/clinids/23.2.255. [DOI] [PubMed] [Google Scholar]
- 53.Raad II, Sabbagh MF. Optimal duration of therapy for catheter-related Staphylococcus aureus bacteremia: a study of 55 cases and review. Clin Infect Dis. 1992;14:75–82. doi: 10.1093/clinids/14.1.75. [DOI] [PubMed] [Google Scholar]
- 54.Musher DM, Lamm N, Darouiche RO, Young EJ, Hamill RJ, Landon GC. The current spectrum of Staphylococcus aureus infection in a tertiary care hospital. Medicine. 1994;73:186–208. doi: 10.1097/00005792-199407000-00002. [DOI] [PubMed] [Google Scholar]
- 55.Libman H, Arbeit RD. Complications associated with Staphylococcus aureus bacteremia. Arch Intern Med. 1984;144:541. [PubMed] [Google Scholar]
- 56.Bone RC. Gram-positive organisms and sepsis. Arch Intern Med. 1994;154:26–34. [PubMed] [Google Scholar]
- 57.Lowy FD. Antimicrobial resistance: the example of Staphylococcus aureus. J Clin Invest. 2003;111:1265–1273. doi: 10.1172/JCI18535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rammelkamp CH, Maxon T. Resistance of Staphylococcus aureus to the action of penicillin. Exp Biol M. 1942;51:386–389. [Google Scholar]
- 59.Jevons MP, Coe AW, Parker MT. Methicillin resistance in staphylococci. Lancet. 281:904–907. doi: 10.1016/s0140-6736(63)91687-8. [DOI] [PubMed] [Google Scholar]
- 60.Chambers HF. Methicillin resistance in staphylococci: molecular and biochemical basis and clinical implications. Clin Microbiol Rev. 1997;10:781–791. doi: 10.1128/cmr.10.4.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Katayama Y, Ito T, Hiramatsu K. A new class of genetic element, staphylococcus cassette chromosome mec, encodes methicillin resistance in Staphylococcus aureus. Antimicrob Agents Ch. 2000;44:1549–1555. doi: 10.1128/aac.44.6.1549-1555.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lim D, Strynadka NC. Structural basis for the β lactam resistance of PBP2a from methicillin-resistant Staphylococcus aureus. Nat Struct Mol Biol. 2002;9:870–876. doi: 10.1038/nsb858. [DOI] [PubMed] [Google Scholar]
- 63.Hooper DC. Fluoroquinolone resistance among Gram-positive cocci. Lancet Infect Dis. 2002;2:530–538. doi: 10.1016/s1473-3099(02)00369-9. [DOI] [PubMed] [Google Scholar]
- 64.Ng EY, Trucksis M, Hooper DC. Quinolone resistance mutations in topoisomerase IV: relationship to the flqA locus and genetic evidence that topoisomerase IV is the primary target and DNA gyrase is the secondary target of fluoroquinolones in Staphylococcus aureus. Antimicrob Agents Ch. 1996;40:1881–1888. doi: 10.1128/aac.40.8.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cormier R, Burda WN, Harrington L, et al. Studies on the antimicrobial properties of N-acylated ciprofloxacins. Bioorg Med Chem Lett. 2012;22:6513–6520. doi: 10.1016/j.bmcl.2012.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hiramatsu K, Aritaka N, Hanaki H, et al. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet. 1997;350:1670–1673. doi: 10.1016/S0140-6736(97)07324-8. [DOI] [PubMed] [Google Scholar]
- 67.Smith TL, Pearson ML, Wilcox KR, et al. Emergence of vancomycin resistance in Staphylococcus aureus. New Engl J Med. 1999;340:493–501. doi: 10.1056/NEJM199902183400701. [DOI] [PubMed] [Google Scholar]
- 68.Cui L, Murakami H, Kuwahara-Arai K, Hanaki H, Hiramatsu K. Contribution of a thickened cell wall and its glutamine nonamidated component to the vancomycin resistance expressed by Staphylococcus aureus Mu50. Antimicrob Agents Ch. 2000;44:2276–2285. doi: 10.1128/aac.44.9.2276-2285.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hiramatsu K. Vancomycin-resistant Staphylococcus aureus: a new model of antibiotic resistance. Lancet Infect Dis. 2001;1:147–155. doi: 10.1016/S1473-3099(01)00091-3. [DOI] [PubMed] [Google Scholar]
- 70.Rolain JM, Abat C, Brouqui P, Raoult D. Worldwide decrease in methicillin resistant Staphylococcus aureus: do we understand something? Clin Microbiol Infect. 2015;21:515–517. doi: 10.1016/j.cmi.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 71.Chabot MR, Stefan MS, Friderici J, Schimmel J, Larioza J. Reappearance and treatment of penicillin-susceptible Staphylococcus aureus in a tertiary medical centre. J Antimicrob Chemother. 2015;70(12):3353–3356. doi: 10.1093/jac/dkv270. [DOI] [PubMed] [Google Scholar]
- 72.Miller BA, Chen LF, Sexton DJ, Anderson DJ. Comparison of the burdens of hospital-onset, healthcare facility-associated Clostridium difficile infection and of healthcare-associated infection due to methicillin-resistant Staphylococcus aureus in community hospitals. Infect Cont Hosp Ep. 2011;32:387–390. doi: 10.1086/659156. [DOI] [PubMed] [Google Scholar]
- 73.Dubourg G, Okdah L, Le Page S, Rolain JM, Raoult D. In vitro activity of ‘old antibiotics’ against highly resistant Gram-negative bacteria. Int J Antimicrob Agents. 2015;46(6):718–720. doi: 10.1016/j.ijantimicag.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 74.Gould IM. The clinical significance of methicillin-resistant Staphylococcus aureus. J Hosp Infect. 2005;61(4):277–282. doi: 10.1016/j.jhin.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 75.Dancer SJ. The real cost of MRSA. In: Gould IM, van der Meer JWM, editors. Antibiotic Policies: Theory and Practice. New York, NY: Springer-Verlag; 2004. pp. 281–309. [Google Scholar]
- 76.Novick RP, Ross HF, Projan SJ, Kornblum J, Kreiswirth B, Moghazeh S. Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J. 1993;12:3967–3975. doi: 10.1002/j.1460-2075.1993.tb06074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gravet A, Couppie P, Meunier O, et al. Staphylococcus aureus isolated in cases of impetigo produces both epidermolysin A or B and LukE-LukD in 78% of 131 retrospective and prospective cases. J Clin Microbiol. 2001;39:4349–4356. doi: 10.1128/JCM.39.12.4349-4356.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bohach GA, Dinges MM, Mitchell DT, Ohlendorf DH, Schlievert PM. Exotoxins. In: Crossley KB, Archer GL, editors. The Staphylococci in Human Disease. New York, NY: Churchill Livingstone; 1997. pp. 83–111. [Google Scholar]
- 79.Kuypers JM, Proctor RA. Reduced adherence to traumatized rat heart valves by a low-fibronectin-binding mutant of Staphylococcus aureus. Infect Immun. 1989;57:2306–2312. doi: 10.1128/iai.57.8.2306-2312.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Patti JM, Bremell T, Krajewska-Pietrasik D, et al. The Staphylococcus aureus collagen adhesin is a virulence determinant in experimental septic arthritis. Infect Immun. 1994;62:152–161. doi: 10.1128/iai.62.1.152-161.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shaw L, Golonka E, Potempa J, Foster SJ. The role and regulation of the extracellular proteases of Staphylococcus aureus. Microbiology. 2004;150:217–228. doi: 10.1099/mic.0.26634-0. [DOI] [PubMed] [Google Scholar]
- 82.Berglund C. Molecular epidemiology of methicillin-resistant Staphylococcus aureus: epidemiological aspects of MRSA and the dissemination in the community and in hospitals [Doctoral thesis] Örebro, Sweden: Örebro University; 2008. [Google Scholar]
- 83.Bubeck Wardenburg J, Schneewind O. Vaccine protection against Staphylococcus aureus pneumonia. J Exp Med. 2008;205:287–294. doi: 10.1084/jem.20072208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cifrian E, Guidry AJ, Bramley AJ, Norcross L, Bastida-Corcuera FD, Marquardt WW. Effect of staphylococcal β toxin on the cytotoxicity, proliferation and adherence of Staphylococcus aureus to bovine mammary epithelial cells. Vet Microbiol. 1996;48:187–198. doi: 10.1016/0378-1135(95)00159-x. [DOI] [PubMed] [Google Scholar]
- 85.Gillet Y, Issartel B, Vanhems P, et al. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immuno-competent patients. Lancet. 2002;359:753–759. doi: 10.1016/S0140-6736(02)07877-7. [DOI] [PubMed] [Google Scholar]
- 86.Otto M. Phenol-soluble modulins. Int J Med Microbiol. 2014;304:164–169. doi: 10.1016/j.ijmm.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Crossley KB, Archer GL. The Staphylococci in Human Disease. 1st ed. New York, NY: Churchill Livingstone Inc; 1997. [Google Scholar]
- 88.Peacock SJ, Moore CE, Justice A, et al. Virulent combinations of adhesin and toxin genes in natural populations of Staphylococcus aureus. Infect Immun. 2002;70:4987–4996. doi: 10.1128/IAI.70.9.4987-4996.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.EFSA The community summary report on trends and sources on zoonoses, zoonotic agents and food-borne outbreaks in European union in 2008. The EFSA J. 2010;8:1496. [Google Scholar]
- 90.Holtfreter S, Grumann D, Schmudde M, et al. Clonal distribution of superantigen genes in clinical Staphylococcus aureus isolates. J Clin Microbiol. 2007;45:2669–2680. doi: 10.1128/JCM.00204-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Smyth CJ, Smyth DS, Kennedy J, Twohig J, Bolton DJ. Staphylococcus aureus: from man or animal-an enterotoxin iceberg. EU-RAIN, Padua, Italy. 2004 Dec 3–4;:85–102. [Google Scholar]
- 92.Tamarapu S, McKillip JL, Drake M. Development of a multiplex polymerase chain reaction assay for detection and differentiation of Staphylococcus aureus in dairy products. J Food Protect. 2001;64:664–668. doi: 10.4315/0362-028x-64.5.664. [DOI] [PubMed] [Google Scholar]
- 93.Podkowik M, Park JY, Seo KS, Bystron J, Bania J. Enterotoxigenic potential of coagulase-negative staphylococci. Int J Food Microbiol. 2013;163:34–40. doi: 10.1016/j.ijfoodmicro.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Le Loir Y, Baron F, Gautier M. Staphylococcus aureus and food poisoning. Genet Mol Res. 2003;2:63–76. [PubMed] [Google Scholar]
- 95.Murray RJ. Recognition and management of Staphylococcus aureus toxin mediated disease. Ann Intern Med. 2005;35:S106–S119. doi: 10.1111/j.1444-0903.2005.00984.x. [DOI] [PubMed] [Google Scholar]
- 96.Clarisse T, Michele S, Olivier T, et al. Detection and quantification of staphylococcal enterotoxin A in foods with specific and sensitive polyclonal antibodies. Food Control. 2013;32:255–261. [Google Scholar]
- 97.Marta D. Molecular Monitoring of Meat Spoiling Pseudomonas Species and Analysis of Staphylococcal Enterotoxin Expression and Formation [PhD thesis] Budapest, Hungary: Corvinus University of Budapest; 2011. [Google Scholar]
- 98.Marrack P, Blackman M, Kushnir E, Kappler J. The toxicity of staphylococcal enterotoxin B in mice is mediated by T cells. J Exp Med. 1990;171:455–464. doi: 10.1084/jem.171.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Oliveira K, Procop GW, Wilson D, Coull J, Stender H. Rapid identification of Staphylococcus aureus directly from blood cultures by fluorescence in situ hybridization with peptide nucleic acid probes. J Clin Microbiol. 2002;40:247–251. doi: 10.1128/JCM.40.1.247-251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Brakstad OG, Aasbakk K, Maeland JA. Detection of Staphylococcus aureus by polymerase chain reaction amplification of the nuc gene. J Clin Microbiol. 1992;30:1654–1660. doi: 10.1128/jcm.30.7.1654-1660.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Trncikova T, Hruskova V, Oravcova K, Pangallo D, Kaclikova E. Rapid and sensitive detection of Staphylococcus aureus in food using selective enrichment and real-time PCR targeting a new gene marker. Food Anal Method. 2009;2:241–250. [Google Scholar]
- 102.Liang H, Cordova SE, Kieft TL, Rogelj S. A highly sensitive immuno-PCR assay for detecting Group A Streptococcus. J Immunol Methods. 2003;279:101–110. doi: 10.1016/s0022-1759(03)00247-3. [DOI] [PubMed] [Google Scholar]
- 103.Barletta JM, Edelman DC, Constantine NT. Lowering the detection limits of HIV-1 viral load using real-time immuno-PCR for HIV-1 p24 antigen. Am J Clin Pathol. 2004;122:20–27. doi: 10.1309/529T-2WDN-EB6X-8VUN. [DOI] [PubMed] [Google Scholar]
- 104.Zhang W, Bielaszewska M, Pulz M, et al. New immuno-PCR assay for detection of low concentrations of Shiga toxin 2 and its variants. J Clin Microbiol. 2008;46:1292–1297. doi: 10.1128/JCM.02271-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Babu D, Muriana PM. Immunomagnetic bead-based recovery and real time quantitative PCR (RT iq-PCR) for sensitive quantification of aflatoxin B1. J Microbiol Meth. 2011;86:188–194. doi: 10.1016/j.mimet.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 106.Flayhart D, Lema C, Borek A, Carroll KC. Comparison of the BBL CHROMagar Staph aureus agar medium to conventional media for detection of Staphylococcus aureus in respiratory samples. J Clin Microbiol. 2004;42:3566–3569. doi: 10.1128/JCM.42.8.3566-3569.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kim HJ, Oh SW. Performance comparison of 5 selective media used to detect Staphylococcus aureus in foods. Food Sci Biotechnol. 2010;19:1097–1101. [Google Scholar]
- 108.Boerema JA, Clemens R, Brightwell G. Evaluation of molecular methods to determine enterotoxigenic status and molecular genotype of bovine, ovine, human and food isolates of Staphylococcus aureus. Int J Food Microbiol. 2006;107:192–201. doi: 10.1016/j.ijfoodmicro.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 109.McMahon WA, Aleo VA, Schultz AM, Horter BL, Lindberg KG. 3M Petrifilm Staph Express Count plate method for the enumeration of Staphylococcus aureus in selected types of meat, seafood, and poultry: collaborative study. J AOAC Int. 2003;86:947–953. [PubMed] [Google Scholar]
- 110.Schoeller NP, Ingham SC. Comparison of the Baird-Parker agar and 3M Petrifilm rapid S. aureus count plate methods for detection and enumeration of Staphylococcus aureus. Food Microbiol. 2001;18:581–587. [Google Scholar]
- 111.Mühlherr JE, Zweifel C, Corti S, Blanco JE, Stephan R. Microbiological quality of raw goat’s and ewe’s bulk-tank milk in Switzerland. J Dairy Sci. 2003;86:3849–3856. doi: 10.3168/jds.S0022-0302(03)73992-7. [DOI] [PubMed] [Google Scholar]
- 112.Smole SC, Aronson E, Durbin A, Brecher SM, Arbeit RD. Sensitivity and specificity of an improved rapid latex agglutination test for identification of methicillin-sensitive and-resistant Staphylococcus aureus isolates. J Clin Microbiol. 1998;36:1109–1112. doi: 10.1128/jcm.36.4.1109-1112.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wichelhaus TA, Kern S, Schäfer V, Brade V, Hunfeld KP. Evaluation of modern agglutination tests for identification of methicillin-susceptible and methicillin-resistant Staphylococcus aureus. Eur J Clin Microbiol Infect Dis. 1999;18:756–758. doi: 10.1007/s100960050396. [DOI] [PubMed] [Google Scholar]
- 114.Berke A, Tilton RC. Evaluation of rapid coagulase methods for the identification of Staphylococcus aureus. J Clin Microbiol. 1986;23(5):916–919. doi: 10.1128/jcm.23.5.916-919.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kwiatek M, Parasion S, Mizak L, Gryko R, Bartoszcze M, Kocik J. Characterization of a bacteriophage, isolated from a cow with mastitis, that is lytic against Staphylococcus aureus strains. Arch Virol. 2012;157:225–234. doi: 10.1007/s00705-011-1160-3. [DOI] [PubMed] [Google Scholar]
- 116.Tsegmed U, Normanno G, Pringle M, Krovacek K. Occurrence of enterotoxic Staphylococcus aureus in raw milk from yaks and cattle in Mongolia. J Food Protect. 2007;70:1726–1729. doi: 10.4315/0362-028x-70.7.1726. [DOI] [PubMed] [Google Scholar]
- 117.Zschöck M, Nesseler A, Sudarwanto I. Evaluation of six commercial identification kits for the identification of Staphylococcus aureus isolated from bovine mastitis. J Appl Microbiol. 2005;98:450–455. doi: 10.1111/j.1365-2672.2004.02470.x. [DOI] [PubMed] [Google Scholar]
- 118.Compernolle V, Verschraegen G, Claeys G. Combined use of Pastorex Staphplus and either of two new chromogenic agars, MRSA ID and CHROMagar MRSA, for detection of methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 2007;45:154–158. doi: 10.1128/JCM.01115-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Graham PL, Lin SX, Larson EL. A US population-based survey of Staphylococcus aureus colonization. Ann Intern Med. 2006;144:318–325. doi: 10.7326/0003-4819-144-5-200603070-00006. [DOI] [PubMed] [Google Scholar]
- 120.Zbinden R, Müller F, Brun F, von Graevenitz A. Detection of clumping factor-positive Staphylococcus lugdunensis by Staphaurex Plus. J Microbiol Meth. 1997;31:95–98. [Google Scholar]
- 121.McKillip JL, Drake M. Real-time nucleic acid–based detection methods for pathogenic bacteria in food. J Food Protect. 2004;67:823–832. doi: 10.4315/0362-028x-67.4.823. [DOI] [PubMed] [Google Scholar]
- 122.Kneifel W, Manafi M, Breit A. Adaptation of two commercially available DNA probes for the detection of E. coli and Staphylococcus aureus to selected fields of dairy hygienean exemplary study. Int J Hyg Envir Med. 1992;192:544–553. [PubMed] [Google Scholar]
- 123.Vernozy-Rozand C, Mazuy-Cruchaudet C, Bavai C, Richard Y. Comparison of three immunological methods for detecting staphylococcal enterotoxins from food. Lett Appl Microbiol. 2004;39:490–494. doi: 10.1111/j.1472-765X.2004.01602.x. [DOI] [PubMed] [Google Scholar]
- 124.Jechorek RP, Johnson RL. Evaluation of the VIDAS staph enterotoxin II (SET 2) immunoassay method for the detection of staphylococcal enterotoxins in selected foods: collaborative study. J AOAC Int. 2008;91:164–173. [PubMed] [Google Scholar]
- 125.Park CE, Akhtar M, Rayman MK. Nonspecific reactions of a commercial enzyme-linked immunosorbent assay kit (TECRA) for detection of staphylococcal enterotoxins in foods. Appl Environ Microb. 1992;58:2509–2512. doi: 10.1128/aem.58.8.2509-2512.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hudson JA. Evaluation of methods for detection of coagulase positive Staphylococcus and staphylococcal toxin in milk and cheese. New Zealand Food Safety Authority. 2010 Feb; Report. [Google Scholar]
- 127.Rose SA, Bankes P, Stringer MF. Detection of staphylococcal enterotoxins in dairy products by the reversed passive latex agglutination (SET-RPLA) kit. Int J Food Microbiol. 1989;8:65–72. doi: 10.1016/0168-1605(89)90081-0. [DOI] [PubMed] [Google Scholar]
- 128.Park CE, Szabo R. Evaluation of the reversed passive latex agglutination (RPLA) test kits for detection of staphylococcal enterotoxins A, B, C, and D in foods. Can J Microbiol. 1986;32:723–727. doi: 10.1139/m86-131. [DOI] [PubMed] [Google Scholar]
- 129.Jensenius JC, Andersen I, Hau J, Crone M, Koch C. Eggs: conveniently packaged antibodies, methods for purification of yolk IgY. J Immunol Methods. 1981;46:63–68. doi: 10.1016/0022-1759(81)90333-1. [DOI] [PubMed] [Google Scholar]
- 130.Minogue TD, Koehler JW, Norwood DA. Targeted next-generation sequencing for diagnostics and forensics. Clin Chem. 2017;63:450–452. doi: 10.1373/clinchem.2016.256065. [DOI] [PubMed] [Google Scholar]
- 131.Wertheim HF, Melles DC, Vos MC, et al. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis. 2005;5:751–762. doi: 10.1016/S1473-3099(05)70295-4. [DOI] [PubMed] [Google Scholar]
- 132.Otto M. Novel targeted immunotherapy approaches for staphylococcal infection. Expert Opin Biol Th. 2010;10:1049–1059. doi: 10.1517/14712598.2010.495115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nomura Y, Yoshinaga M, Masuda K, Takei S, Miyata K. Maternal antibody against toxic shock syndrome toxin-1 may protect infants younger than 6 months of age from developing Kawasaki syndrome. J Infect Dis. 2002;185:1677–1680. doi: 10.1086/340513. [DOI] [PubMed] [Google Scholar]
- 134.Harro CD, Betts RF, Hartzel JS, et al. The immunogenicity and safety of different formulations of a novel Staphylococcus aureus vaccine (V710): results of two Phase I studies. Vaccine. 2012;30:1729–1736. doi: 10.1016/j.vaccine.2011.12.045. [DOI] [PubMed] [Google Scholar]
- 135.Torres VJ, Pishchany G, Humayun M, Schneewind O, Skaar EP. Staphylococcus aureus IsdB is a hemoglobin receptor required for heme iron utilization. J Bacteriol. 2006;188:8421–8429. doi: 10.1128/JB.01335-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.O’Riordan K, Lee JC. Staphylococcus aureus capsular polysaccharides. Clin Microbiol Rev. 2004;17:218–234. doi: 10.1128/CMR.17.1.218-234.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Schaffer AC, Lee JC. Staphylococcal vaccines and immunotherapies. Infect Dis Clin N Am. 2009;23:153–171. doi: 10.1016/j.idc.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 138.Poole-Warren LA, Hallett MD, Hone PW, Burden SH, Farrell PC. Vaccination for prevention of CAPD associated staphylococcal infection: results of a prospective multicentre clinical trial. Clin Nephrol. 1991;35:198–206. [PubMed] [Google Scholar]
- 139.Menzies BE, Kernodle DS. Site-directed mutagenesis of the alpha-toxin gene of Staphylococcus aureus: role of histidines in toxin activity in vitro and in a murine model. Infect Immun. 1994;62:1843–1847. doi: 10.1128/iai.62.5.1843-1847.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wardenburg JB, Schneewind O. Vaccine protection against Staphylococcus aureus pneumonia. J Exp Med. 2008;205:287–294. doi: 10.1084/jem.20072208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fattom A, Fuller S, Propst M, et al. Safety and immunogenicity of a booster dose of Staphylococcus aureus types 5 and 8 capsular polysaccharide conjugate vaccine (StaphVAX®) in hemodialysis patients. Vaccine. 2004;23:656–663. doi: 10.1016/j.vaccine.2004.06.043. [DOI] [PubMed] [Google Scholar]
- 142.Brown EL, Dumitrescu O, Thomas D, et al. The Panton-Valentine leukocidin vaccine protects mice against lung and skin infections caused by Staphylococcus aureus USA300. Clin Microbiol Infect. 2009;15:156–164. doi: 10.1111/j.1469-0691.2008.02648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shinefield H, Black S, Fattom A, et al. Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. New Engl J Med. 2002;346:491–496. doi: 10.1056/NEJMoa011297. [DOI] [PubMed] [Google Scholar]
- 144.Karauzum H, Chen G, Abaandou L, et al. Synthetic human monoclonal antibodies toward staphylococcal enterotoxin B (SEB) protective against toxic shock syndrome. J Biol Chem. 2012;287:25203–25215. doi: 10.1074/jbc.M112.364075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rozemeijer W, Fink P, Rojas E, et al. Evaluation of approaches to monitor Staphylococcus aureus virulence factor expression during human disease. PLoS ONE. 2015;10:e0116945. doi: 10.1371/journal.pone.0116945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Giersing BK, Dastgheyb SS, Modjarrad K, Moorthy V. Status of vaccine research and development of vaccines for Staphylococcus aureus. Vaccine. 2016;34:2962–2966. doi: 10.1016/j.vaccine.2016.03.110. [DOI] [PubMed] [Google Scholar]
- 147.Kuklin NA, Clark DJ, Secore S, et al. A novel Staphylococcus aureus vaccine: iron surface determinant B induces rapid antibody responses in rhesus macaques and specific increased survival in a murine S. aureus sepsis model. Infect Immun. 2006;74:2215–2223. doi: 10.1128/IAI.74.4.2215-2223.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lin L, Ibrahim AS, Avanesian V, et al. Th17 cells are not required for host defense against murine disseminated candidiasis, but are required for vaccine-mediated protection. J Immunol. 2009;182:132.10. [Google Scholar]
- 149.Monteil MA, Kaniuk ASC, Hobbs JR. Staphylococcal opsonization and anti-Staphylococcus aureus IgG subclass antibodies in patients with severe or recurrent S. aureus infections. FEMS Microbiol Lett. 1990;64:259–262. doi: 10.1111/j.1574-6968.1990.tb03527.x. [DOI] [PubMed] [Google Scholar]
- 150.Dryla A, Prustomersky S, Gelbmann D, et al. Comparison of antibody repertoires against Staphylococcus aureus in healthy individuals and in acutely infected patients. Clin Diagn Lab Immun. 2005;12:387–398. doi: 10.1128/CDLI.12.3.387-398.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sreeramoju P, Porbandarwalla NS, Arango J, et al. Recurrent skin and soft tissue infections due to methicillin-resistant Staphylococcus aureus requiring operative debridement. Am J Surg. 2011;201:216–220. doi: 10.1016/j.amjsurg.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 152.David MZ, Mennella C, Mansour M, Boyle-Vavra S, Daum RS. Predominance of methicillin-resistant Staphylococcus aureus among pathogens causing skin and soft tissue infections in a large urban jail: risk factors and recurrence rates. J Clin Microbiol. 2008;46:3222–3227. doi: 10.1128/JCM.01423-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.LeClaire RD, Hunt RE, Bavari S. Protection against bacterial superantigen staphylococcal enterotoxin B by passive vaccination. Infect Immun. 2002;70:2278–2281. doi: 10.1128/IAI.70.5.2278-2281.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Park CE, Akhtar M, Rayman MK. Evaluation of a commercial enzyme immunoassay kit (RIDASCREEN) for detection of staphylococcal enterotoxins A, B, C, D, and E in foods. Appl Environ Microb. 1994;60:677–681. doi: 10.1128/aem.60.2.677-681.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Schmidt CS, White CJ, Ibrahim AS, et al. NDV-3, a recombinant alumadjuvanted vaccine for Candida and Staphylococcus aureus, is safe and immunogenic in healthy adults. Vaccine. 2012;30:7594–7600. doi: 10.1016/j.vaccine.2012.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]