Abstract
Tick-borne encephalitis (TBE) virus, which is usually divided into European, Far Eastern and Siberian subtypes, is a serious public health problem in several European and Asian countries. Vaccination is the most effective measure to prevent TBE; cross-subtype protection elicited by the TBE vaccines is biologically plausible since all TBE virus subtypes are closely related. This manuscript systematically explores available data on the cross-subtype immunogenicity elicited by the currently available Western vaccines based on the European subtype. Completed immunization course of 3 doses of both Western vaccines determined very high seroconversion/seropositivity rates against both Far Eastern and Siberian subtypes among previously flavivirus-naïve subjects. All but one study found no statistically significant difference in titers of neutralizing antibodies against strains belonging to homologous and heterologous subtypes. Pooled analysis of randomized controlled trials on head-to-head comparison of immunogenicity of Western and Russian TBE vaccines did not reveal differences in seroconversion rates against Far Eastern isolates in either hemagglutination inhibition (risk ratio = 0.98, p = 0.83) or enzyme-linked immunosorbent (risk ratio = 0.95, p = 0.44) assays after 2 vaccine doses. This suggests that, in regions where a heterogeneous TBE virus population circulates, vaccines based on the European subtype may be used alongside vaccines based on the Far Eastern subtype. Studies on the field effectiveness of TBE vaccines and investigation of vaccination failures, especially in countries where different subtypes co-circulate, will further elucidate TBE vaccination-induced cross-subtype protection.
Keywords: cross-subtype immunogenicity, European subtype, Far Eastern subtype, Siberian subtype, tick-borne encephalitis, TBE, vaccines, cross-protection
Abbreviations
- C
capside
- CEE
Central European encephalitis
- CI
confidence interval
- d
day
- E
envelope
- ELISA
enzyme-linked immunosorbent assay
- FSME
Frühsommer-Meningoenzephalitis [German] (tick-borne encephalitis)
- GMT
geometric mean titer
- HI
hemagglutination inhibition
- IFA
indirect immunofluorescence
- IgG
Immunoglobulin G
- IPVE
Institute of Poliomyelitis and Viral Encephalitis
- M
membrane
- μNT
microneutralization test
- NR
not reported
- NS
non-structural
- NT
neutralization test
- prM
pre-membrane
- RCT
randomized controlled trial
- RNA
ribonucleic acid
- RR
risk ratio
- RSSE
Russian spring summer encephalitis virus
- SCR
seroconversion rate
- SD
standard deviation
- SMD
standardized mean difference
- SPR
seropositivity rate
- TBE
tick-borne encephalitis
- TBEV
tick-borne encephalitis virus
- TBEV-Eu
European subtype of TBEV
- TBEV-FE
Far Eastern subtype of TBEV
- TBEV-Sib
Siberian subtype of TBEV
- VIEU
Vienna unit
- we
week
- WHO
World Health Organization
- y
year
Introduction
Tick-borne encephalitis (TBE) is a serious international public health problem, being endemic to a large geographic area, which extends from North-Eastern France and Scandinavia to North-Eastern China and Northern Japan; moreover, international travel to these regions has increased markedly.1,2 The causative agent of TBE, the TBE virus (TBEV), belongs to the genus Flavivirus of the family Flaviviridae.3 The genome of flaviviruses is constituted by single-stranded positive-sense RNA; the single open reading frame encodes 3 structural (C – capside, prM/M – pre-membrane/membrane and E – envelope) and 7 non-structural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B and NS5).4 Protein E is the main target of TBEV-neutralizing antibodies5; proteins prM, NS1 and NS3 have also been shown to be immunogenic.6
TBEV is usually divided into 3 subtypes: European (TBEV-Eu), Far Eastern (TBEV-FE) and Siberian (TBEV-Sib); the first subtype is mainly transmitted by Ixodes ricinus, while TBEV-FE and TBEV-Sib are chiefly associated with Ixodes persulcatus.1,2,7-9 As has been shown by Ecker et al.,10 despite the wide geographic distribution of TBEV, the degree of variation in amino acid sequence within subtypes is low and does not exceed 2.2%, while the variation between subtypes is 3.6–5.6%. However, in a more recent publication, Pogodina et al.11 reported a greater difference in amino acid sequences (up to 9%) and variability of up to 17.3% at the nucleotide level.11 Moreover, it has been suggested that 2 strains, namely 178–79 and 886-84, isolated in the Irkutsk region in Eastern Siberia, could constitute independent genotypes;12 subsequent complete genome sequencing has revealed that these strains adjoin TBEV-FE.13 The existence of more than 3 subtypes was subsequently confirmed by Demina et al.14, who concluded that subtype 4 is represented by a single strain 178–79, while the fifth subtype includes the strain 886–84 and another 9 isolates forming the so-called “886 group.” A detailed analysis of distance and phylogenetic analysis performed by Grard et al.15 has led to a novel taxonomic proposal of a single species of TBEV which includes 4 distinct types: Louping ill virus (with Spanish, British and Irish subtypes), Western TBEV (corresponding to TBEV-Eu), Turkish sheep encephalitis virus with the Greek goat encephalitis virus subtype, and Eastern TBEV, which comprises TBEV-FE and TBEV-Sib. In the present paper, however, we will adopt the more widely used classification into the 3 main subtypes, namely TBEV-Eu, TBEV-FE and TBEV-Sib.
Active immunization is the most effective means of preventing TBE.1,2 Four different TBE vaccines are currently on the market: FSME-Immun (Baxter, Austria), Encepur (Novartis vaccines, Germany), EnceVir (the Tomsk branch of the Federal State Unitary Enterprise “Mikrogen," Russia) and another Russian vaccine from the Institute of Poliomyelitis and Viral Encephalitis – IPVE vaccine – also called TBE vaccine Moscow.1,2,7,9 Pediatric formulations of FSME-Immun and Encepur,1,2 as well the recently licensed pediatric vaccine Kleshch-E-Vak (Tick-E-Vac)16 produced by the IPVE, are available. A pediatric formulation of EnceVir is under clinical development.17 Since their introduction, these vaccines have undergone several modifications aimed at improving their immunogenicity, safety and tolerability profiles (e.g., historic versions of FSME-Immun containing thiomersal, an albumin-free formulation known as TicoVac, and a polygeline-stabilized formulation of Encepur); vaccine antigens, however, have not changed. The vaccines are similar, in that they are all based on cell-cultured killed whole TBEV, adjuvanted with Al(OH)3 and produced in accordance with World Health Organization (WHO) Good Manufacturing Practices. On the other hand, these vaccines contain different amounts of antigen and use 4 different strains: Western vaccines are based on TBEV-Eu (Neudörfl strain in FSME-Immun and strain K23 in Encepur), while Russian vaccines are based on TBEV-FE (Sofjin strain in IPVE and Tick-E-Vak vaccines, and strain 205 in EnceVir).1,2,7,9 Numerous clinical studies have revealed excellent immunogenicity, safety and tolerability profiles of all these vaccines17-26 and have demonstrated their field effectiveness in regions with a high vaccination coverage.27,28 A locally used Chinese vaccine based on the Senzhang TBEV-FE strain also exists; this vaccine has successfully passed phase I, II, and III trials,29 data from which, however, are not available in the international literature.2,7 Moreover, steady progress in biotechnology has prompted researchers to explore other approaches to obtaining effective TBE vaccines, including RepliVax-TBE candidates,30 live chimeras,31 and naked DNA vaccines,32,33 which are all under development.
The question of whether TBE vaccines based on TBEV-Eu strains can also provide protection against strains belonging to TBEV-FE and TBEV-Sib is of crucial interest for several reasons. First, in several regions, comprising a large area from the northern Baltic to the Urals, Ixodes ricinus overlaps with Ixodes persulcatus;34 this leads to the co-circulation of the 3 TBEV subtypes in countries such as the Baltic States35 and in some regions in the European part of Russia.36 Second, there is documented evidence of the geographical expansion of non-European subtypes, as has been observed in Finland, where 11 TBEV-Sib strains have been isolated.37 Third, international travel has substantially increased to TBE endemic areas2 where a vaccinated person may be exposed to a subtype heterologous to the TBEV vaccine subtype. Fourth, in Russia, in addition to the 2 Russian vaccines, both Western vaccines have been registered (FSME-Immun in 1993 and Encepur in 1998), and the question of their ability to provide cross-subtype protection has arisen in Siberia and the Far East, where non-European TBEV subtypes circulate.38 And finally, a limited protection of all licensed vaccines against some strains belonging to TBEV-Sib has recently been emphasized.39
Given the above-mentioned considerations, the present study had 3 main goals: (1) to gather and explore published data on the feasibility of cross-subtype immunogenicity elicited by the currently available Western vaccines; (2) to compare vaccine immunogenicity toward homologous and heterologous subtypes, and (3) to establish whether TBEV-Eu-based Western vaccines are as effective as TBEV-FE-based Russian vaccines against Far Eastern isolates. To address research question 2, we examined studies on vaccine immunogenicity to isolates belonging to both homologous and heterologous subtypes, while with regard to research question 3, we analyzed only head-to-head randomized controlled trials (RCTs) which investigated the immunogenicity of at least one Western and at least one Russian vaccine against any strain belonging to TBEV-FE. It should be noted that, regarding research questions 2 and 3, the meta-analytical approach was adopted whenever possible. However, if single studies did not report any comparison between vaccines and/or TBEV subtypes/strains, appropriate statistical tests were applied.
Results
Of the 745 citations (739 and 6 retrieved through automatic and manual searches, respectively), 14 papers17,40-52 met the inclusion criteria (Fig. 1). Of these, 4 were single-center RCTs,17,41,42,49 4 were derived from a single cohort study in which cross-subtype immunogenicity was measured at different time-points up to 7 y after the primary vaccination course (Leonova GN, personal communication),45,47,48,50 and 2 papers described the immunological effectiveness of TBE vaccines during a mass immunization program in Sverdlovsk Oblast (Russia);46,52 the other studies were observational and serological. The characteristics of the studies included are reported in Table 1. Eight papers were in Russian17,41,42,45,46,49,50,52 and the remaining 6 were in English. Most studies (n = 10) were carried out in Russia,17,41,42,45-50,52 2 in Japan,43,44 and the remaining 2 in the European Union.40,51 The immunogenicity of the Austrian vaccine was evaluated in 7 studies,17,41-44,49,51 that of the German vaccine in 5,40,45,47,50,52 and that of both vaccines in 2.46,48 In 8 studies, participants received 3 doses,40,43,45-48,51,52 while a 2-dose regimen was investigated in 5 papers.17,41,42,44,49 Only one study50 evaluated the immune response to heterologous subtypes in remote periods after the primary vaccination course (up to 7 years) and after a booster administered 7 y after the third dose. Most studies evaluated a conventional vaccination schedule;40-48,50,52 the rapid schedule of FSME-Immun was examined in 3 studies.17,49,51 Pediatric formulations were evaluated in 2 Russian papers.17,52 Throughout the studies, the age of participants ranged from 15 months52 to 70 y.49
Figure 1.
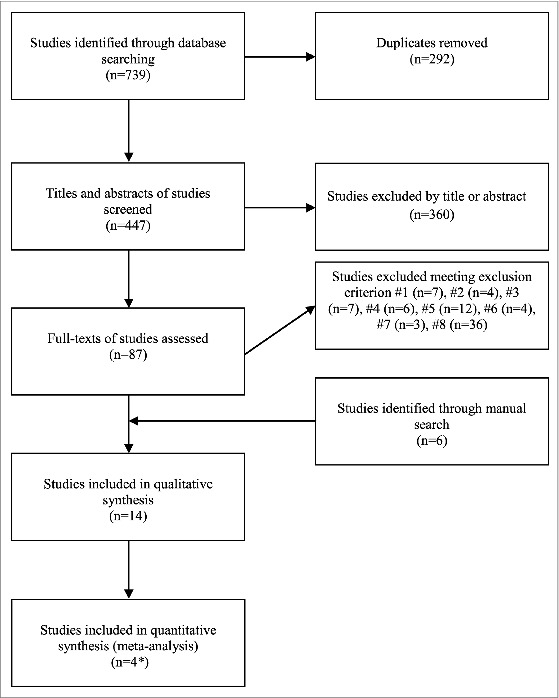
Flowchart of the study selection process. Since 2 studies41,42 used a commercial hemagglutination inhibition (HI) kit based on strain 139, 1 study17 used an enzyme-linked immunosorbent assay (ELISA) kit based on strain 205 and another study49 used both commercial kits, 2 separate meta-analyses on the basis of serological assay were performed.
Table 1.
Characteristics of studies included.
| Study first author, year, location [ref] | Vaccine used | N of subjects on enrollment | Age | N of doses (schedule) | Serum collection after vaccination | TBEV subtypes and strains tested | Immunological assay used | Immunological outcomes reported | Notes |
|---|---|---|---|---|---|---|---|---|---|
| Klockmann et al., 1991, Germany [40] | Encepur | 17 | 20-50 y | 3 (0-1-11 mo) | 4 we after 3rd dose | TBEV-FE: Sofjin TBEV-Eu: 10 strains |
NT | Minimum, median, maximum titers; GMT | Protection against Louping ill virus was also tested. TBEV-Eu strains tested: K23, IX10, Hypr, Trpisovsky, Petracova, 274/II, Gbelce, Cg1, Dobrostan, Absettarov |
| Vorobyeva et al., 1996, Russia [41] | 1. FSME-Immun; 2. IPVE |
1. 50 2. 50 |
18-23 y | 2 (0-2 mo) | 1 mo after 2nd dose | TBEV-FE: 139 TBEV-Eu: Neudörfl |
HI, ELISA IgG | SCR in HI (≥1:10) and ELISA IgG (≥1:32 VIEU at serum dilution 1:50) | Results of serological tests are reported for only 30 subjects immunized with FSME-Immun and 35 subjects in IPVE group. Immune response against strain 139 was measured by HI commercial kit, while that against strain Neudörfl was measured by commercial ELISA IgG kit. |
| Pavlova et al., 1999, Russia [42] | 1. FSME-Immun 2. IPVE |
1. 107 2. 116 |
7-17 y | 2 (0-4 mo) | 4 we after 2nd dose | TBEV-FE: 139 | HI | SCR (4-fold increase), log10 GMT | The 2nd was administered to 95 subjects in FSME-Immun group and to 106 subjects in IPVE group. HI was performed by using only 147 paired serum samples, 2 of which were seropositive on enrollment and were excluded. Immune response against strain 139 was measured by HI commercial kit. |
| Chiba et al., 1999, Japan [43] | FSME-Immun | 10 | 24-53 y | 3 (0-4we-1y) | 4 we after each dose | TBEV-FE: Oshima 5-10, Sofjin TBEV-Eu: Hochosterwitz |
NT | SPR (≥1:20), individual titers and GMT | 2 subjects were seropositive to Japanese encephalitis virus and therefore excluded. Only 4 subjects received 3 doses. |
| Hayasaka et al., 2001, Japan [44] | FSME-Immun | 11 | 23-53 y | 2 (0-4we) | 4 we after 2nd dose | TBEV-FE: KH98-5, VL99-m11 TBEV-Sib: IR99-2f7 TBEV-Eu: Hochosterwitz |
NT | SPR (≥1:20), individual titers | - |
| Pavlenko et al., 2005, Russia [45]* | Encepur | 145 | 17-68 y | 3 (0-4we-1y) | 1 mo after 1st dose, 1 and 11 mo after 2nd dose and 1 mo after 3rd dose | TBEV-FE: P-73, P-202, P-69, 139 | HI, IFA | SPR (unclear threshold titer, probably ≥1:10), GMT | 28 subjects had IgG antibodies to TBEV on enrollment and were excluded. Immune response against strains P-73, P-202 and P-69 was measured in both HI and IFA. Immune response against strain 139 was measured by HI commercial kit. |
| Prochorova et al., 2006, Russia [46] | 1. Encepur 2. FSME-Immun 3. IPVE 4. EnceVir |
1. 121 2. 125 3. 123 4. 103 |
1. 7-8 y 2. 12-13 y 3. 12-13 y 4. 18-20 y |
3 (conventional schedule) | 1 mo after each dose | TBEV-FE: 205 | ELISA IgG | SPR (≥1:100) | 2, 7, 4, 28 subjects in FSME-Immun, Encepur, IPVE, EnceVir groups, respectively, had IgG antibodies to TBEV on enrollment and were excluded. ELISA IgG was performed by using commercial kit produced by “Vektor-Best” and is based on strain 205. |
| Leonova et al., 2007, Russia [47]* | Encepur | 44 | 26-68 y | 3 (0-4we-1y) | 1 y after 2nd dose and 1 mo after 3rd dose | TBEV-FE: P-73, P-69, P-202, 205 | NT, ELISA IgG | SPR in NT (≥1:10) and ELISA IgG (according to instructions, probably ≥1:100), GMT | Immune response against strains P-73, P-69 and P-202 was measured by NT, while that against strain 205 was measured by ELISA IgG commercial kit. |
| Leonova et al., 2009, Russia [48]* | 1. Encepur 2. FSME-Immun 3. IPVE 4. EnceVir |
1. 6/11 2. 51/32 3. 30/17 4. 17/56 |
1. 43.0±6.3 y§ 2. 33.4±2.9 y§ 3. 43.4±4.9 y§ 4. 45.6±3.2y§ |
3 (0-4we-1y) | a. 2-5 mo after 3rd dose b. 2 y after 3rd dose |
TBEV-FE: P-73, 205 | NT, ELISA IgG, IFA | SCR in NT (≥1:10), ELISA IgG (according to instructions, probably ≥1:100), GMT | Immune response against strain P-73 was measured by NT, while that against strain 205 was measured by ELISA IgG commercial kit. Results of IFA are reported in a pooled form, indistinctly for subjects who received different vaccines (comprising Russian ones), and were not recorded. |
| Shutova et al., 2009, Russia [49] | 1. FSME-Immun; 2. EnceVir |
1. 50 2. 50 |
18-70 y | 2 (0-14 d) | 14 d after 1st dose and 14, 30 d after 2nd dose | TBEV-FE: 205 and 139 | HI, ELISA IgG | SCR (4-fold increase), seroprotection rate (unclear threshold titer, probably ≥1:100), log10 GMT | 11 subjects in FSME-Immun group and 21 in EnceVir group had anti-TBEV antibodies and were excluded. Immune response against strain 139 was measured by HI commercial kit, while that against strain 205 was measured by commercial ELISA IgG kit (“Vektor-Best”). Seroprotection rate was reported only 1 mo post-dose 2. |
| Pavlenko et al., 2011, Russia [50]* | Encepur | 20 | NR | 3+1 (0-4we-1y-7y) | 1 mo, 3 y, 5 y and 7 y after 3rd dose and 1 mo after a booster | TBEV-FE: P-73, 205 TBEV-Sib: Kolarovo-2008 |
NT, ELISA IgG | SCR in NT (≥1:10), ELISA IgG (according to instructions, probably ≥1:100), GMT | Booster dose was administered to 15 subjects. Immune response against strain P-73 was measured by NT, while that against strain 205 was measured by ELISA IgG commercial kit. |
| Orlinger et al., 2011, Belgium [51] | FSME-Immun | 41 | 16-65 y | 3 (0, 12±2 d, ≈12 mo) | 21st d after the 3rd dose | TBEV-FE: Sofjin, Oshima 5-10; TBEV-Sib: Vasilchenko TBEV-Eu: Neudörfl, K23 |
μNT | SPR (probably ≥1:16), mean μNT titer | Tested strains were all hybrid viruses in which the prM and E sequences of the West Nile virus strain NY99 were exchanged with the corresponding TBEV sequences. Protection against Omsk hemorrhagic fever virus was also tested. |
| Ankudinova et al., Russia, 2013 [52] | Encepur Children | 140 | 15-40 mo | 3 (0, 29±1 d, 300 d) | 2 we and 9 mo after the 2nd dose and 3 we after the 3rd dose | TBEV-FE: 205 | ELISA IgG | SCR (4-fold increase), seroprotection rate (≥1:100), GMT | 4 children had IgG antibodies to TBEV on enrollment; 68 children did not undergo blood collection and/or 2nd and/or 3rd dose, and were excluded. |
| Feldblium et al., 2013, Russia [17] | 1. FSME-Immun Junior; 2. EnceVir children formulation |
1. 44 2. 42 |
3-17 y | 2 (0-14 d) | 14 d after 1st dose and 1 and 6 mo after 2nd dose | TBEV-FE: 205 | ELISA IgG | SCR (4-fold increase), GMTs | ELISA IgG was performed by using commercial kit produced by “Vektor-Best” and is based on strain 205. |
Notes: *The same cohort of vaccinees was followed-up and cross-subtype immunogenicity was measured at different time-points by means of different assays (Leonova GN, personal communication).
§Combined age of 2 independent cohorts. d, day; ELISA, enzyme-linked immunosorbent assay; HI, hemagglutination inhibition; IFA, indirect immunofluorescence assay; GMT, geometric mean titer; mo, month; NR, not reported; NT, neutralization test; SCR, seroconversion rate; SPR, seropositivity rate; TBEV, tick-borne encephalitis virus; TBEV-Eu, European subtype of TBEV; TBEV-FE, Far Easter subtype of TBEV; TBEV-Sib, Siberian subtype of TBEV; VIEU, Vienna unit; we, week; y, year.
The immune response against subtypes heterologous to vaccine subtype was most frequently measured by means of a neutralization test (NT) (7 studies)40,43,44,47,48,50,51 and heterologous ELISA IgG (enzyme-linked immunosorbent assay) (7 studies),17,46-50,52 followed by hemagglutination inhibition (HI) (4 studies)41,42,45,49 and indirect immunofluorescence assay (IFA) (1 study).45 Six papers41,45,47-50 described vaccination-induced immunogenicity measured by more than one assay. ELISA IgG was performed by means of a commercially available kit (Joint Stock Company Vector-Best, Novosibirsk, Russia), which is based on strain 205 belonging to TBEV-FE. HI was also performed by means of a commercial kit (NPO Virion branch of NPO Mikrogen Tomsk, Russia) based on TBEV-FE strain 139.
Most frequently, immunogenicity was assessed within one month post-vaccination. Overall, 12 heterologous strains were tested; of these, 9 belonged to TBEV-FE (KH98-5, Oshima 5–10, P-69, P-73, P-202, Sofjin, VL99-m11, 139 and 205) and 3 to TBEV-Sib (IR99-2f7, Kolarovo-2008 and Vasilchenko). All but one strain (Oshima 5–10) were isolated in the Asian part of Russia.
Immune response elicited by FSME-immun and Encepur against TBEV-FE and TBEV-Sib strains
Ten studies17,41-47,49,52 reported data on immunogenicity after 2 doses (Table 2). Generally, 2 doses of both vaccines elicited considerable humoral immune responses against heterologous subtypes. However, as demonstrated by both seroconversion/seropositivity rates (SCRs/SPRs) and the reciprocal of geometric mean titers (GMTs), immune response against single strains varied. For instance, Leonova et al.47 found statistically lower NT SPRs on using strain P-69 than on using P-73 and P-202 Far Eastern isolates.
Table 2.
Immune response against heterologous subtypes after 2 doses of Western TBE vaccines.
| Assay | Vaccine | Time after 2nd dose, months | Strain | GMT | Sero-outcome % (N/Total) | Ref |
|---|---|---|---|---|---|---|
| NT | FSME-Immun | 1 | Oshima 5–10* | 40.0§¶ | 87.5 (7/8)¶ | [43] |
| Sofjin* | 47.6§¶ | 100 (8/8)¶ | ||||
| KH98–5* | 37.6§ | 100 (11/11) | [44] | |||
| VL99-m11* | 62.2§ | 100 (11/11) | ||||
| IR99–2f7** | 58.4§ | 100 (11/11) | ||||
| Encepur | 12 | P-73* | 19.7 | 56.8 (25/44) | [47] | |
| P-202* | 15 | 52.3 (23/44) | ||||
| P-69* | 11.3 | 27.3 (12/44) | ||||
| ELISA IgG | FSME-Immun | 0.5 | 205* | 309 | 71.8 (28/39) | [49] |
| 1 | NR | 91.6 (109/119) | [46] | |||
| 524.8 | 97.4 (38/39) | [49] | ||||
| 692.4 | 72.7 (32/44) | [17] | ||||
| 6 | 919.4 | 100 (44/44) | ||||
| Encepur | 0.5 | 66 | 72.1 (49/68)/35.3 (24/68)^ | [52] | ||
| 1 | NR | 39.8 (47/118) | [46] | |||
| 9 | 3 | 20.6 (14/68)/4.4 (3/68)^ | [52] | |||
| 12 | NR | 40.9 (18/44) | [47] | |||
| HI | FSME-Immun | 0.5 | 139* | 15.1 | 79.5 (31/39) | [49] |
| 1 | 79.4 | 100 (75/75) | [42] | |||
| NR | 83.3 (25/30) | [41] | ||||
| 15.8 | 84.6 (33/39) | [49] | ||||
| Encepur | 1 | 139* | 8 | 62.9# | [45] | |
| P-73* | 32 | 94.3# | ||||
| P-202* | 37 | 92.4# | ||||
| P-69* | 15 | 71.4# | ||||
| 11 | 139* | 9 | NR¤ | |||
| P-73* | 12 | 82.4# | ||||
| P-202* | 11.3 | 84.6# | ||||
| P-69* | 11.3 | NR¤ | ||||
| IFA | Encepur | 1 | P-73* | 60 | NR¤ | |
| P-202* | 52 | NR¤ | ||||
| P-69* | 42 | NR¤ | ||||
| 11 | P-73* | 19.7 | NR¤ | |||
| P-202* | 16 | NR¤ | ||||
| P-69* | 10.6 | NR¤ |
Notes:
*Far Eastern subtype.
**Siberian subtype.
§Calculated values.
¶Two subjects had anti-Japanese encephalitis virus antibodies and were excluded; the authors reported GMT of 44 and 43 against Oshima 5–10 and Sofjin strains, respectively.
^Subjects with IgG titer ≥1:100/subjects with a 4-fold increase in IgG titers. On enrollment, 117 subjects were TBEV-naïve; however, it is unclear how many serum samples were collected at each time-point.
¤only figures are available. ELISA, enzyme-linked immunosorbent assay; HI, hemagglutination inhibition; IFA, indirect immunofluorescence assay; GMT, geometric mean titer; NR, not reported; NT, neutralization test.
Most subjects who received a full vaccination course of 3 doses were seropositive against all heterologous strains of both subtypes (Table 3). NT was most frequently used; indeed, one paper48 reported a higher sensitivity of NT than ELISA, although SCRs determined by the 2 assays generally matched. As in the case of post-dose 2, there was some degree of variation in the level of antibody titers against single heterologous strains. This again involved strain P-69; only one third of vaccinees proved seropositive and GMT levels were relatively low (1:28).47
Table 3.
Immune response against heterologous subtypes after 3 doses of Western TBE vaccines.
| Assay | Vaccine | Time after 3rd dose, months | Strain | GMT | Sero-outcome % (N/Total) | Ref |
|---|---|---|---|---|---|---|
| NT | FSME-Immun | 0.75 | Sofjin* | 319§ | 100 (41/41) | [51] |
| Oshima 5–10* | 409§ | 100 (41/41) | ||||
| Vasilchenko** | 224§ | 100 (41/41) | ||||
| 1 | Oshima 5–10* | 80.0¶^ | 100 (3/3)^ | [43] | ||
| Sofjin* | 63.5¶^ | 100 (3/3)^ | ||||
| 2–5 | P-73* | 112 | 88.2 (45/51) | [48] | ||
| 24 | P-73* | 34 | 78.1 (25/32) | |||
| Encepur | 1 | Sofjin* | 56 | 100 (17/17)# | [40] | |
| P-73* | 128 | 95.5 (42/44) | [47] | |||
| P-202* | 34 | 97.7 (43/44) | ||||
| P-69* | 28 | 63.6 (28/44) | ||||
| Kolarovo-2008** | 208 | 100 (20/20) | [50] | |||
| 2–5 | P-73* | 91 | 100 (6/6) | [48] | ||
| 24 | 42 | 100 (11/11) | ||||
| 36 | 49 | 100 (19/19) | [50] | |||
| 60 | 45 | 100 (14/14) | ||||
| 84 | 39 | 100 (15/15) | ||||
| 36 | Kolarovo-2008** | 140 | 100 (19/19) | |||
| 60 | 140 | 100 (14/14) | ||||
| 84 | 112 | 100 (15/15) | ||||
| ELISA IgG | FSME-Immun | 1 | 205* | NR | 96.6 (115/119) | [46] |
| 2–5 | NR | 86.3 (44/51) | [48] | |||
| 24 | NR | 75.0 (24/32) | ||||
| Encepur | 0.75 | 205* | 592 | 92.6 (63/68)/79.4 (54/68)¤ | [52] | |
| 1 | NR | 94.9 (112/118) | [46] | |||
| NR | 93.2 (41/44) | [47] | ||||
| 2–5 | NR | 83.3 (5/6) | [48] | |||
| 24 | NR | 100 (11/11) | ||||
| 36 | 1940 | 100 (19/19) | [50] | |||
| 60 | 1380 | 100 (14/14) | ||||
| 84 | 1120 | 93.3 (14/15) | ||||
| HI | Encepur | 1 | 139* | 39 | 98.8¶¶ | [45] |
| P-73* | 45 | 100¶¶ | ||||
| P-202* | 39 | 100¶¶ | ||||
| P-69* | 15 | 100¶¶ | ||||
| IFA | Encepur | 1 | P-73* | 49 | 100¶¶ | |
| P-202* | 45 | 98.8¶¶ | ||||
| P-69* | 37 | 98.8¶¶ |
Notes:
*Far Eastern subtype.
**Siberian subtype.
§Mean microneutralization titers are reported.
¶Calculated values.
^One subject had anti-JEV antibodies and was excluded.
#No SCR is reported; however, minimum NT titer against Sofjin strain was 21.
¤Subjects with IgG titer ≥1:100/subjects with a 4-fold increase in IgG titers.
¶¶On enrollment, 117 subjects were TBEV-naïve; however, it is unclear how many serum samples were collected at each time-point. ELISA, enzyme-linked immunosorbent assay; HI, hemagglutination inhibition; IFA, indirect immunofluorescence assay; GMT, geometric mean titer; NR, not reported; NT, neutralization test.
The rapid immunization schedule was found to be highly immunogenic against all subtypes.17,49,51 On microneutralization assay (μNT), all 41 subjects vaccinated with 3 doses of FSME-Immun were seropositive against 2 TBEV-FE strains (Sofjin and Oshima 5–10) and the Vasilchenko strain belonging to TBEV-Sib. In comparison with the TBEV-FE strains, the mean μNT titer against the Vasilchenko strain was lower (1:319/1:409 and 1:224); the difference, however, was not statistically significant.51 In one Russian study49 the rapid schedule of FSME-Immun was investigated by means of ELISA and HI after 2 doses administered 2 weeks apart. On ELISA one month after the 2nd dose, almost all subjects (38 of 39) proved to have seroconverted; on HI, this proportion was slightly lower (33 of 39). The rapid protocol of FSME-Immun Junior was also highly immunogenic in children and adolescents, with up to 100% seroconversion following 2 doses.52
A progressive waning of antibody titers against both TBEV-FE and TBEV-Sib was reported in a study50 in which 15 subjects, who had previously been immunized with 3 doses of Encepur according to the conventional schedule, were followed up for 7 y. Although SCRs remained 100%, the authors observed about 4.7- and 1.9-fold reductions in GMTs (from 1 month to 7 y after the 3rd dose) of neutralizing antibodies against strains P-73 and Kolarovo-2008 (from 1:182 to 1:39 and from 1:208 to 1:112, respectively). Similar results were documented by means of ELISA IgG, with a nearly 2.5-fold decrease (from 1:2750 to 1:1120) in GMTs. A subsequent booster increased GMTs by about 3.5 times on NT against both heterologous strains (post-booster GMTs against P-73 and Kolarovo-2008 strains of 1:138 and 1:471, respectively). A similar pattern was noted on ELISA, with GMTs rising to the level of one month post-dose 3 (1:2750).
Comparison of immunogenicity against European and non-European subtypes
In 5 studies40,41,43,44,51 the humoral immune response was tested against strains belonging to both heterologous subtypes and homologous TBEV-Eu subtype. Across all 5 studies, SCRs/SPRs against homologous and heterologous subtypes were similarly high. Specifically, in 2 studies43,51 after completion of the primary vaccination course of 3 doses (both conventional and rapid schedule) and in one study44 after 2 doses of FSME-Immun, seropositivity against homologous and heterologous subtypes was documented in all vaccinees. Chiba et al.43 also reported high SPRs (NT ≥ 1:20): 100% (n = 8) against the Hochosterwitz TBEV-Eu strain and the Sofjin TBEV-FE strain, and 87.5% against the Oshima 5–10 TBEV-FE strain after 2 FSME-Immun doses. In one Russian study,41 which also assessed a 2-dose regimen of FSME-Immun, 86.7% of vaccines proved to have seroconverted on ELISA based on the Neudörfl strain, while 83.3% had seroconverted on HI based on TBEV-FE strain 139. Similarly, 3 doses of Encepur were able to induce an NT titer >1:20 against the Sofjin TBEV-FE strain and against 10 different European isolates.40
GMTs or individual titers were reported in 3 studies,40,43,44 while in one paper51 mean μNT titers were calculated (Table 4). Of these studies, only in 243,51 were statistical tests performed to compare antibody titers against different strains. Neither study found a significant difference at the 5% level; however, the results of statistical tests were not reported.
Table 4.
Comparison of neutralizing antibody titers against homologous and heterologous subtypes reported in single studies.
| Vaccine | N of doses | Antibody titer against TBEV-FE or TBEV-Sib strains | Antibody titer against TBEV-Eu strains | Difference† | Ref |
|---|---|---|---|---|---|
| FSME-Immun | 2 | Oshima 5–10: 40.0§¶* Sofjin: 47.6§¶* |
Hochosterwitz : 87.2§¶* | Kruskal-Wallis test: H = 4.55, p = 0.10 | [43] |
| IR99–2f7: 58.4§* KH98–5: 37.6§* VL99-m11: 62.2§* |
Hochosterwitz: 96.7§* | Kruskal-Wallis test: H = 5.02, p = 0.17 | [44] | ||
| 3 | Oshima 5–10: 80.0§^* Sofjin: 63.5§^* |
Hochosterwitz: 127.0§^* | Kruskal-Wallis test: H = 2.29, p = 0.32 | [43] | |
| Sofjin: 319** Oshima 5–10: 409** Vasilchenko: 224** |
Neudörfl: 360** K 23: 338** |
ANOVA: F = 2.05, p = 0.089 | [51] | ||
| Encepur | 3 | Sofjin: 56* | K 23: 307*; IX 10: 134*; Hypr: 110*; Trpisovsky: 114*; Petracova: 129*; 274/II: 110*; Gbelce: 98*; Cg 1: 55*; Dobrostan: 31*; Absettarov: 46* | ANOVA: F = 16.94, p < 0.001. Tukey post test: least significant difference in mean loge titers at 5% level is 0.69. Mean loge titer against Sofjin strain is significantly lower than those against 4 of 10 TBEV-Eu strains (K23, IX 10, Trpisovsky and Petracova) | [40] |
Notes:
†Comparison was performed since none of the papers had reported results of statistical tests.
§Calculated values.
¶Two subjects had anti-Japanese encephalitis virus antibodies and were excluded; the authors reported GMTs of 44, 43 and 65 against Oshima 5–10, Sofjin and Hochosterwitz strains, respectively.^ One subject had anti-JEV antibodies and was excluded.
*Geometric mean titers are reported.
**Mean microneutralization titers are reported. TBEV-Eu, European subtype of TBEV; TBEV-FE, Far Eastern subtype of TBEV; TBEV-Sib, Siberian subtype of TBEV.
As shown in Table 4, in only one study40 was a significant variation observed in neutralizing antibody levels against single strains, with 4 significant post-hoc multiple comparisons between prototype Sofjin and TBEV-Eu isolates (Table 4).
Owing to the presence of raw data in only 2 studies, and considering between-study differences in methodology, the strains analyzed and their numbers, we decided not to pool titers of neutralizing antibodies.
Head-to-head RCTs on the immunogenicity of Western and Russian TBE vaccines
Four RCTs17,41,42,49 compared the immunogenicity of Russian and Western vaccines. As shown in Table 5, 3 trials were classified as being at unclear risk of bias, while the fourth RCT was judged to be at high risk of bias owing to reporting bias (pre-specified outcome of seroprotection was not reported in the results). Inter-rater agreement was substantial (K: 0.63, 95% CI: 0.35 – 0.92).
Table 5.
Judgments on risk of bias in each trial included.
| Risk of bias |
||||
|---|---|---|---|---|
| Domain | Vorobjeva et al. 1996 [41] | Pavlova et al. 1999 [42] | Shutova et al. 2009 [49] | Feldblium et al. 2013 [17] |
| Random sequence generation | Unclear | Unclear | Unclear | Unclear |
| Allocation concealment | Unclear | Unclear | Unclear | Unclear |
| Blinding of participants | Unclear | Unclear | Unclear | Unclear |
| Blinding of personnel | Unclear | Unclear | Unclear | Unclear |
| Blinding of outcome assessment | Unclear | Unclear | Low | Unclear |
| Incomplete outcome data | Unclear | Unclear | Low | Low |
| Selective reporting | Low | Low | Low | High |
| Other bias | Unclear | Unclear | Unclear | Unclear |
All studies used FSME-Immun as a Western vaccine, while the comparator Russian vaccine was IPVE in 2 studies41,42 and EnceVir in another 2 studies.17,49 Children's formulations of FSME-Immun and EnceVir were compared in one trial.17 In all studies, the immunogenicity of vaccines was evaluated after 2 doses, while one of the 4 studies49 reported SCRs after each of 3 doses. Two studies41,42 used a commercial HI kit based on strain 139; one study17 used an ELISA kit based on strain 205, while the fourth study49 used both HI and ELISA commercial kits. Two RCTs evaluated conventional schedules,41,42 while the other 2 studies17,49 evaluated rapid schedules.
Vorobjeva et al.41 found no significant difference in SCRs on HI after 2 doses of FSME-Immun and IPVE (FSME-Immun: 83.3% [n = 30], IPVE: 91.4% [n = 35]). By contrast, among previously seronegative children and adolescents, SCR after 2 doses was higher in the FSME-Immun group than in the IPVE group (FSME-Immun: 100% [n = 75], IPVE: 91.4% [n = 70]) as reported by Pavlova et al.42 On ELISA, Shutova et al.49 recorded higher SCRs among subjects vaccinated with EnceVir than among FSME-Immun vaccinees (FSME-Immun: 71.8% [n = 39], EnceVir: 100% [n = 29]) 2 weeks after the 2nd dose. However, this difference disappeared one month after the 2nd dose (97.4% and 100%). HI performed by the same authors revealed similar SCRs at both time-points (FSME-Immun: 79.5% and 84.6%, EnceVir: 89.7% and 93.1%, respectively). Likewise, on ELISA one month after 2 doses of FSME-Immun Junior and the pediatric formulation of EnceVir, each administered 2 weeks apart, SCRs were very similar (FSME-Immun: 72.7% [n = 44], EnceVir: 78.6% [n = 42]), reaching 100% for both vaccines 6 months after the 2nd dose.17
Three studies41,42,49 reported SCRs on HI on sera collected one month after the 2nd dose, and 2 studies17,49 reported SCRs on ELISA one month after 2 doses. We therefore performed 2 separate pooled analyses. In the analysis of HI SCRs, a substantial level of heterogeneity was observed, which justified the use of a random effects model. As shown in Figure 2, no significant association (p = 0.83) emerged between SCRs on HI after 2 doses of FSME-Immun or the Russian vaccines. It can be seen that a probable source of the heterogeneity observed is the trial by Pavlova et al.,42 which showed a significantly higher SCR in subjects immunized with FSME-Immun than in those immunized with IPVE vaccine. Notably, the study population in this trial consisted of 7–17-year-olds, while vaccinees in other 2 trials were adults. Removal of this trial from the meta-analysis completely reset heterogeneity (I2 = 0%, Q = 0 [p = 0.98]) without, however, altering the pattern of the pooled effect (Mantel-Haenszel risk ratio [RR] = 0.91 [95% CI: 0.80 – 1.03], p = 0.14).
Figure 2.
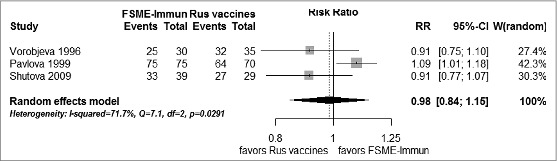
Forest plot of seroconversion rates on hemagglutination inhibition assay by means of commercial kit one month after 2 doses of FSME-Immun and Russian vaccines: random effects model.
The second pooled analysis did not find any such heterogeneity (I2 = 0%, Q = 0.55 [p = 0.46]); no difference emerged in SCRs determined by ELISA among vaccinees who had received 2 doses of EnceVir or FSME-Immun 2 weeks apart (Mantel-Haenszel RR = 0.95 [95% CI: 0.84 – 1.08], p = 0.44). Adding the results of ELISA after 2 doses of FSME-Immun and EnceVir, as obtained from a field effectiveness study by Prochorova et al.,46 did not alter the pooled estimates (Mantel-Haenszel [RR] = 1.02 [95% CI: 0.94 – 1.10], p = 0.68).
Owing to the paucity of studies, we did not check for publication bias, nor could sensitivity analysis be performed.
The HI titers one month post-dose 2 reported by Pavlova et al.42 did not differ significantly between subjects immunized with FSME-Immun and those immunized with IPVE: standardized mean difference (SMD) = 0.15 (95% CI: -0.18 – 0.48), p = 0.37. SMD in antibody titers determined on HI by Shutova et al.49 was significantly lower among FSME-Immun vaccinees than EnceVir vaccinees only one month after the 2nd vaccine dose (SMD = -0.95 [95% CI: -1.45 – −0.44] P < 0.001) but not 2 weeks after the 2nd dose, when the sign change in SMD was noted (SMD = 0.45 [95% CI: -0.04 – 0.94] p = 0.069). We do not report the results of the pooled analysis on HI titers one month post-dose 2 recorded in these 2 trials because of the unacceptably high heterogeneity level (I2 = 92.1%; Q = 12.65, P < 0.001).
Two trials17,49 reported the GMTs determined on ELISA after 2 doses of FSME-Immun and EnceVir administered 2 weeks apart. One49 found that significantly lower titers were elicited by FSME-Immun than by EnceVir both 2 and 4 weeks post-dose 2 (SMD = −2.94 [95% CI: −3.64 – −2.24] P < 0.001 and SMD = −1.20 [95% CI:−1.73 – −0.68] P < 0.001, respectively). By contrast, the trial by Feldblium et al.17 did not find any significant difference between FSME-Immun Junior and the pediatric formulation of EnceVir either one or 6 months post-dose 2 (SMD = 0.31 [95% CI: −0.11 – 0.74] p = 0.15 and SMD = 0.01 [95% CI: -0.41 – 0.43] p = 0.96). Again, no pooled analysis of ELISA titers was done owing to the high heterogeneity (I2 = 94.8%; Q = 19.40, P < 0.001).
Discussion
This paper provides a comprehensive review of the cross-subtype protection elicited by both currently available Western vaccines. These findings could be used for future research in the field. The main strength of our investigation is that we systematically searched the Russian language literature. Indeed, most of the papers and all the head-to-head RCTs included in the review were published in Russian; this is not surprising since both IPVE and EnceVir are marketed only in the Russian Federation and some post-Soviet countries. The inclusion of non-English literature may make a review more comprehensive, increase the precision of pooled estimates and reduce systematic errors53,54; moreover, it has also been shown that the need to include non-English papers may depend on the topic of the review.55 In the case of TBE, the inclusion of Russian studies is particularly appropriate for several reasons: all vaccines are commercialized in Russia,1,2 the TBEV population is very heterogeneous,36 the study of TBE has a long history, dating back to its description by Silber et al.,56 and the first “brain-made vaccine” was prepared in the former Soviet Union.38
Cross-subtype protection provided by the currently available TBE vaccines is biologically plausible, since the 3 main subtypes are closely related both genetically and antigenically.1 The grading of scientific evidence in support of the hypothesis of vaccine-induced cross-protection has been estimated in a WHO position paper;57 this included 5 studies,44,47,48,51,58 and attributed a final score of 2 (out of 4), concluding that the currently available vaccines protect against all 3 subtypes, though suggesting that the true effect may be substantially different from the estimated effect.
To answer our research questions, we were able to identify a higher number of studies. With regard to the SCRs/SPRs against TBEV-FE, the protection given by the Western vaccines against almost all the strains tested may be judged adequate. In 2 Japanese papers,43,44 all subjects had NT titers of 1:20 or higher against 5 heterologous isolates; an NT titer of 20 was later shown to be the lower threshold of protective IgG activity.59 The only exception concerns TBEV-FE strain P-69, for which only one third of vaccinees were seropositive after 3 doses of Encepur. It is noteworthy that this strain, which was isolated from the blood of a tick-exposed healthy subject, forms a separate subclade within TBEV-FE.47 Similarly high immune responses have been found against Siberian isolates, with seropositivity rates as high as 100%.44,50,51 However, in human studies, only 3 TBEV-Sib strains were tested. Indeed, the small number of strains tested is cited in the WHO position paper as a serious limitation in the designs of the studies examined.57
In most studies examining titers of neutralizing antibodies against homologous and heterologous strains, no statistically significant difference among tested strains was established, even though NT titers against heterologous subtypes tended to be lower. It should, however, be noted that all these studies involved few samples, and probably had low statistical power, since the minimum number of vaccinees required in order to observe a clinically significant difference was not calculated. The only exception concerns the relatively old study by Klockmann et al.,40 who observed a significant variation in GMTs against 11 TBEV isolates; this was attributed by the authors to the presence of minor differences in neutralizing epitopes of single isolates. This explanation is reasonable since, for example, TBE vaccination-induced μNT titers against Omsk hemorrhagic fever virus, which is more distantly related to TBEV, have proved to be significantly lower than those against TBEV strains.51 Moreover, in the study by Hayasaka et al.,44 the lowest NT titers were those against TBEV-FE strain KH98-5, while the highest GMTs among heterologous strains were documented against TBEV-Sib strain IR99-2f7. Notably, the amino acid sequence of the E protein of the strain KH98-5 differs from the Neudörfl vaccine strain by 23 amino acids (n = 496), while the difference between IR99-2f7 and Neudörfl strains concerns 16 amino acids (n = 496) (determined by means of the BLAST program in GenBank,60 data not shown). In later papers5,51 it was suggested that similar inconsistencies in the immune the response to different strains may have been due to a poorly standardized methodology. To overcome that problem, a novel test system was proposed which enabled unbiased head-to-head comparison of the humoral response against the 3 subtypes by constructing hybrid viruses. These hybrids were created by using the West Nile virus backbone and encoding prM and E structural proteins of single TBEV isolates. According to the authors, this method enabled discrepancies in viral growth and infectivity to be mitigated, while preserving the antigenic characteristics of single wild isolates. Indeed, the 2 studies5,51 (one was a murine model5 not included in this paper) that used this approach found similar patterns of vaccine-induced cross-immunogenicity between homologous and heterologous strains.
Another important issue concerning the TBEV-FE and TBEV-Sib isolates tested is their correspondence to the currently circulating TBEV population. Indeed, of 12 heterologous strains reported in the present paper, 5 were identified more than 40 y ago, while only one (Kolarovo-2008) was isolated less than 10 y ago. This has been emphasized,39 since Zausaev-like Siberian strains have prevailed in Russia in recent years, but no human studies on the immunogenicity provided by the inactivated vaccines against these strains have been conducted. In their murine model, Morozova et al.39 documented only partial protection of all inactivated vaccines against the Siberian strain 2086 isolated in 2010. Further research on the immunogenicity of vaccines against modern TBEV isolates is therefore needed.
The fact that very high cross-subtype immunogenicity was provided by the rapid schedule of the Western vaccines (as demonstrated in 3 studies)17,49,51 is of particular importance in a globalized world, as this modality may be suitable for short-term travelers to TBEV-endemic zones where non-European subtypes circulate (such as the Baltic states, Russia, Mongolia, Northern China or Hokkaido). Indeed, international tourist arrivals to Central/Eastern Europe and North East Asia are steadily rising, reaching more than 230 million in 2012.61
Our third research question regarded the direct comparison of Russian and Western vaccines in terms of immunogenicity against Far Eastern isolates. This question was posed with a double aim. First, it could demonstrate the efficacy of the Western vaccines against TBEV-FE; second, the question is of a certain local significance (in Russia) as both FSME-Immun and Encepur are on the market in regions where TBEV-FE circulates. Evidence of moderate quality suggests that TBEV-Eu-based vaccines are as effective as TBEV-FE-based ones against Far Eastern isolates. The use of standardized commercial ELISA and HI kits in selected RCTs enables the above-mentioned methodological issues to be avoided. On the other hand, the results of our pooled analysis should be interpreted cautiously on account of the small number of trials included, the limited number of participants in single RCTs and the moderate methodological quality of these trials. Indeed, the results of meta-analyses of small trials may not be confirmed by subsequent large RCTs for at least 2 reasons: publication bias and the limited methodological quality of pooled studies. Thus, small trials tend to be accepted for publication if they find a statistically significant intervention effect.62 Moreover, Vickers et al.63 found a high proportion of positive results among acupuncture trials conducted in Russia/the former Soviet Union and some Asian countries. Although we were not able to check formally for publication bias, its presence seems to be unlikely, since all 4 RCTs concluded that Russian and Western vaccines displayed almost equal immunological performance. Furthermore, it has been shown that the methodological quality of non-English clinical trials may be lower than that of those published in English.55 We believe that sub-optimal methodological quality observed stems from the later adoption of standards of reporting trials in Russia.
In addition to the above-mentioned limitations regarding the small numbers of participants in single studies, the moderate quality of trials and a certain risk of country-related publication bias, the present paper may suffer from other shortcomings. Specifically, most studies that compared immunogenicity against European and non-European subtypes (research question 2) were observational and thus prone to the selection bias. Moreover, to answer our research question 3, we identified only a few RCTs and thus were not able to perform sensitivity analysis nor meta-regression. Finally, although the results reported in the selected RCTs were almost consistent, their moderate quality could reduce the value of the pooled estimates.
In conclusion, to date there is no universally accepted standardized serological correlate of protection against TBE, and all studies on the efficacy of vaccination are based on immunogenicity rather than clinical protection.64 Indeed, it has been underlined that the in vitro presence of neutralizing antibodies against multiple viruses does not guarantee cross-protection against all these viruses in vivo.65 Improvements in TBE surveillance, field studies on the effectiveness of TBE vaccines and the investigation of vaccination failures, especially in countries where different subtypes co-circulate, will further provide useful insights into TBEV vaccination-induced cross-subtype protection.
Methods
Search strategy
To identify eligible studies, the following international databases were systematically searched: PubMed, Web of Science and Scopus. Russian language literature was systematically searched by using the scientific electronic database eLIBRARY.RU, which is the largest information portal in Russia.
In order to ensure maximal retrieval, free text searching was undertaken. In PubMed, the following search syntax was composed: (TBE OR TBEV OR tick-borne encephalitis OR Central European encephalitis OR CEE OR Russian spring summer encephalitis OR RSSE) AND (vaccin* OR immunis* OR immuniz* OR FSME-Immun OR TicoVac OR Encepur) AND (cross-protect* OR ((heterologous OR heterotypic OR cross-subtype) AND protection) OR cross-neutraliz* OR cross-neutralis* OR cross-immun* OR cross-react* OR neutraliz* OR neutralis*). In Scopus and Web of Science, the same entry terms were searched for in “title, abstract, keyword – TITLE-ABS-KEY” and “topic – TS,” respectively. Owing the smaller number of Russian language papers, research on the eLIBRARY.RU was performed by using the simpler free text searching “клещевой энцефaлйт & вaкцйн*” (“tick-borne encephalitis and vaccin*”). Additionally, a manual search was performed by scanning reference lists of identified studies; Google Scholar was further used in order to identify papers that cited the selected items.
As the development of FSME-Immun – the oldest vaccine currently marketed – began in the early 1970s,66 the search was restricted to studies published from January 1970 onwards. The last search was performed on 3rd April 2014.
Study eligibility
Studies of any design which assessed the immunogenicity elicited by the Western TBE vaccines against at least one TBEV-FE or TBEV-Sib strain were eligible. Both historic and current formulations of FSME-Immun and Encepur were considered, since the vaccine antigens have not changed.1,2,7 Study populations were restricted to healthy flavivirus-naïve subjects of any age vaccinated with at least 2 doses of FSME-Immun or Encepur. The outcome of interest was the humoral immune response to heterologous subtypes, as measured by any serological assay. If an unclear TBEV strain was used in serological assays, the corresponding author of that study was contacted by email. Full-text articles published in English, French, Italian or Russian were eligible.
In the present paper, the unit of analysis was a single serological test; therefore, when the immunologic response was investigated at different time-points and/or by means of different serological assays, but the results were presented in more than one publication, these papers were not considered redundant. By contrast, papers presenting the results of serological assays performed at the same time-points and using sera from the same subjects were deemed redundant. Anyway, if the same research group had produced several similar publications, the senior author was contacted personally for further explanation.
Exclusion criteria were formulated as follows: (1) reviews, commentaries, opinion publications without original data, papers without numeric data; (2) articles published in languages other than English, French, Italian or Russian; (3) redundant publications; (4) animal studies; (5) investigation of only Russian, candidate or obsolete TBE/Langat-based vaccines; (6) explicit statement on documented evidence of TBE or any flavivirus infection or studies in which some participants had anti-TBEV antibodies on enrollment but there were no separate data for seropositive and seronegative subjects; (7) explicit statement on history of yellow fever and/or Japanese encephalitis vaccination; (8) the immune response elicited by Western vaccines was measured only against strains belonging to TBEV-Eu or other members of the mammalian tick-borne flavivirus group or against mosquito-borne flaviviruses.
Data extraction
Data from eligible studies were extracted by 2 independent reviewers (AD and EKA) and inserted into an ad hoc table, which included the following information: study design, sample size, age of vaccinees, study location, vaccine, number of doses and vaccination schedule, time of serum collection post-immunization, serological assay, TBEV subtypes (both homologous and heterologous) and strains tested, immunogenicity against single strains.
Studies reporting either individual or group data were eligible. If immunogenicity was measured after each successive immunization, data recorded after the 2nd, 3rd or booster doses were extracted separately. If more than one immunologic assay was performed, the results of each were recorded. We were compelled to use a composite outcome for serological response67 since some studies reported SCRs, SPRs and seroprotection rates after vaccination. The definition of each sero-outcome, if provided by the authors, was extracted. Raw or summarized antibody titers (GMTs, log-transformed GMTs or mean titers), if available, were recorded from each study.
Quality assessment of RCTs
To assess the methodological quality of the RCTs selected, the Cochrane Collaboration's tool for assessing risk of bias68 was used; this assesses risk of bias (defined as low, unclear and high) in domains of sequence generation and allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other sources of bias. As suggested by the Cochrane Collaboration,68 the summary assessment outcome of single studies was classified as low (low risk of bias in all domains), unclear (unclear risk of bias in ≥1 domain but no domains at high risk of bias), or high (at least one domain with high risk of bias) risk of bias. Assessment was performed independently by 2 authors (AD and EKA); any disagreement was solved by consensus.
Statistical analysis
We compared the immune response to different subtypes in single studies which investigated immunogenicity against both homologous and heterologous strains but did not report the results of statistical tests. When individual titers were available, the Kruskal-Wallis test was used to evaluate differences among 3 or more strains. Since we used a non-parametric test, a simplistic approach for treating censored observations was adopted: left-censored at dilution titer values of antibody titers (for example, <20) were treated as substitute values expressed as half of the detection limit; right-censored titers, such as ≥640, were set to the maximum dilution specified in the article.69 When only mean titers and their standard deviations (SDs) or standard errors were available, one-way analysis of variance (ANOVA) was performed. A Tukey's multiple-comparisons test was used to detect differences in log-transformed NT titers against single strains.
Skewed continuous outcomes (GMTs) were treated in accordance with the recommendations of the United States Advisory Committee on Immunization Practices70 by converting GMTs and their SDs to the natural logarithm (loge) scale. In studies in which GMTs ± SD were expressed on log10 scale, we first took their anti-log10 and then reconverted them to the loge scale. For studies presenting a range (sample minimum and maximum) of titers, SDs were imputed by dividing the range by 4.71 The SMD with 95% CIs was used to quantify the differences in loge-transformed means of antibody titers elicited by Russian and Western vaccines. We planned to pool SMDs of single studies by using Hedges' adjusted g.
The meta-analysis of binary outcomes was performed in order to pool SCRs reported in RCTs on head-to-head comparison of Russian and Western vaccines. Pooled results were expressed as RRs with 95% CIs. In all pooled analyses, random-effects models weighted by the DerSimonian-Laird method were first performed; however, when observed heterogeneity was low (I2 < 40%), fixed-effects models using the Mantel-Haenszel method for weighting were re-applied. Meta-analysis was not done when heterogeneity was too high (I2 > 85%).72 We planned to assess potential publication bias by means of funnel plot and Harbord's test. We also planned a priori to perform a leave-one-out sensitivity analysis, in order to ascertain that the estimates were not driven by single trials, and meta-regression to identify study characteristics that were associated with between-study heterogeneity (such as study quality).
Inter-rater agreement in assessing the methodological quality of RCTs was quantified by means of Cohen's K, which was interpreted as follows: ≤0 – poor, 0–0.20 – slight, 0.21–0.40 – fair, 0.41-0.60 – moderate, 0.61–0.80 – substantial, 0.81–1.0 – almost perfect.73
All data were analyzed by means of the R stats package, version 3.0.1.74
Disclosure of Potential Conflicts of Interest
A.D., D.P., E.K.A., A.S., R.G., and D.A. declare that they have no competing interests. U.A. is an employee of Baxter S.p.A.
References
- 1.World Health Organization (WHO) Vaccines against tick-borne encephalitis: WHO position paper. Wkly Epidemiol Rec 2011; 86:241-56; PMID:21661276 [PubMed] [Google Scholar]
- 2.Amicizia D, Domnich A, Panatto D, Lai PL, Cristina ML, Avio U, Gasparini R. Epidemiology of tick-borne encephalitis (TBE) in Europe and its prevention by available vaccines. Hum Vaccin Immunother 2013; 9:1163-71; PMID:23377671; http://dx.doi.org/ 10.4161/hv.23802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.International Committee on Taxonomy of Viruses Virus taxonomy: 2011 release. Available at: http://www.ictvonline.org/virusTaxonomy.asp?version=2011&bhcp=1 Accessed: November 17, 2014 [Google Scholar]
- 4.McMinn PC. The molecular basis of virulence of the encephalitogenic flaviviruses. J Gen Virol 1997; 78(Pt 11):2711-22; PMID:9367356 [DOI] [PubMed] [Google Scholar]
- 5.Fritz R, Orlinger KK, Hofmeister Y, Janecki K, Traweger A, Perez-Burgos L, Barrett PN, Kreil TR. Quantitative comparison of the cross-protection induced by tick-borne encephalitis virus vaccines based on European and Far Eastern virus subtypes. Vaccine 2012; 30:1165-9; PMID:22178103; http://dx.doi.org/ 10.1016/j.vaccine.2011.12.013 [DOI] [PubMed] [Google Scholar]
- 6.Putnak R. Progress in the development of recombinant vaccines against dengue and other arthropod-borne flaviviruses. In: Kurstak E, ed. Modern Vaccinology. New York: Plenum Medical, 1994; 231-52. [Google Scholar]
- 7.Kollaritsch H, Paulke-Korinek M, Holzmann H, Hombach J, Bjorvatn B, Barrett A. Vaccines and vaccination against tick-borne encephalitis. Expert Rev Vaccines 2012; 11:1103-19; PMID:23151167; http://dx.doi.org/ 10.1586/erv.12.86 [DOI] [PubMed] [Google Scholar]
- 8.Lindquist L, Vapalahti O. Tick-borne encephalitis. Lancet 2008; 371:1861-71; PMID:18514730; http://dx.doi.org/ 10.1016/S0140-6736(08)60800-4 [DOI] [PubMed] [Google Scholar]
- 9.Donoso Mantke O, Escadafal C, Niedrig M, Pfeffer M, Working group for tick-borne encephalitis virus C. Tick-borne encephalitis in Europe, 2007 to 2009. Euro Surveill 2011; 16:pii:19976; PMID:21968423 [DOI] [PubMed] [Google Scholar]
- 10.Ecker M, Allison SL, Meixner T, Heinz FX. Sequence analysis and genetic classification of tick-borne encephalitis viruses from Europe and Asia. J Gen Virol 1999; 80(Pt 1):179-85; PMID:9934700 [DOI] [PubMed] [Google Scholar]
- 11.Pogodina VV, Karan' LS, Koliasnikova NM, Levina LS, Malenko GV, Gamova EG, Lesnikova MV, Kiliachina AS, Esiunina MS, Bochkova NG, et al. [Evolution of tick-borne encephalitis and a problem of evolution of its causative agent]. Vopr Virusol 2007; 52:16-21. Russian; PMID:18041219 [PubMed] [Google Scholar]
- 12.Zlobin VI, Demina TV, Belikov SI, Butina TV, Gorin OZ, Adel'shin RV, Grachev MA. [Genetic typing of TBEV strains in terms of homology analysis of an envelope protein's gene fragment]. Vopr Virusol 2001; 1:17-22. Russian [PubMed] [Google Scholar]
- 13.Karan' LS, Braslavskaya SI, Myazin AE. [Development of detection methods and genotyping of TBE virus in terms of amplification technologies]. Vopr Virusol 2007; l6:17-22. Russian [PubMed] [Google Scholar]
- 14.Demina TV, Dzhioev YP, Verkhozina MM, Kozlova IV, Tkachev SE, Plyusnin A, Doroshchenko EK, Lisak OV, Zlobin VI. Genotyping and characterization of the geographical distribution of tick-borne encephalitis virus variants with a set of molecular probes. J Med Virol 2010; 82:965-76; PMID:20419810; http://dx.doi.org/ 10.1002/jmv.21765 [DOI] [PubMed] [Google Scholar]
- 15.Grard G, Moureau G, Charrel RN, Lemasson JJ, Gonzalez JP, Gallian P, Gritsun TS, Holmes EC, Gould EA, de Lamballerie X. Genetic characterization of tick-borne flaviviruses: new insights into evolution, pathogenetic determinants and taxonomy. Virology 2007; 361:80-92; PMID:17169393; http://dx.doi.org/ 10.1016/j.virol.2006.09.015 [DOI] [PubMed] [Google Scholar]
- 16. Federal State Unitary Enterprise on Manufacture of Bacterial and Viral Preparations of Chumakov Institute of Poliomyelitis and Viral Encephalitides, Russian Academy of Medical Sciences; News. Available at: http://www.chumakovs.ru/about_enterprise/news/detailed/russkaya_versiya/novosti_ot_14122012/ Accessed: November 17, 2014. [Google Scholar]
- 17.Feldblium IV, Menshikova MG, Danilina TV, Okuneva IA, Perminova OA, Semerikov VV, Pavroz KA. [Evaluation of reactogenicity, safety and immunogenicity of domestic vaccine “EnceVir” with a reduced antigen load in immunization of children 3-17 years old accordingly to emergency vaccination protocol]. Epdemiol Infekts Bolezn 2013; 4:53-7. Russian [Google Scholar]
- 18.Schöndorf I, Beran J, Cizkova D, Lesna V, Banzhoff A, Zent O. Tick-borne encephalitis (TBE) vaccination: applying the most suitable vaccination schedule. Vaccine 2007; 25:1470-5; PMID:17196713; http://dx.doi.org/ 10.1016/j.vaccine.2006.10.028]Z [DOI] [PubMed] [Google Scholar]
- 19.Zent O, Banzhoff A, Hilbert AK, Meriste S, Słuzewski W, Wittermann Ch. Safety, immunogenicity and tolerability of a new pediatric tick-borne encephalitis (TBE) vaccine, free of protein-derived stabilizer. Vaccine 2003; 21:3584-92; PMID:12922086; http://dx.doi.org/ 10.1016/S0264-410X(03)00421-3 [DOI] [PubMed] [Google Scholar]
- 20.Zent O, Beran J, Jilg W, Mach T, Banzhoff A. Clinical evaluation of a polygeline-free tick-borne encephalitis vaccine for adolescents and adults. Vaccine 2003; 21:738-41; PMID:12531352; http://dx.doi.org/ 10.1016/S0264-410X(02)00592-3 [DOI] [PubMed] [Google Scholar]
- 21.Ehrlich HJ, Pavlova BG, Fritsch S, Poellabauer EM, Loew-Baselli A, Obermann-Slupetzky O, Maritsch F, Cil I, Dorner F, Barrett PN. Randomized, phase II dose-finding studies of a modified tick-borne encephalitis vaccine: evaluation of safety and immunogenicity. Vaccine 2003; 22:217-23; PMID:14615149; http://dx.doi.org/ 10.1016/S0264-410X(03)00563-2 [DOI] [PubMed] [Google Scholar]
- 22.Loew-Baselli A, Konior R, Pavlova BG, Fritsch S, Poellabauer E, Maritsch F, Harmacek P, Krammer M, Barrett PN, Ehrlich HJ, et al. FSME-IMMUN study group Safety and immunogenicity of the modified adult tick-borne encephalitis vaccine FSME-IMMUN: results of two large phase 3 clinical studies. Vaccine 2006; 24:5256-63; PMID:16624457; http://dx.doi.org/ 10.1016/j.vaccine.2006.03.061 [DOI] [PubMed] [Google Scholar]
- 23.Ehrlich HJ, Loew-Baselli A, Poellabauer EM, Fritsch S, Mai I, Pavlova BG, Maritsch F, Domer F, Behre U, Barrett PN. Randomized, phase II, multicenter dose-finding studies of a modified tick-borne encephalitis vaccine in children: evaluation of safety and immunogenicity of two vaccinations with FSME-Immun® new. Int J Med Microbiol 2004; 293(Suppl 37):126-7; PMID:1461514914615149 [Google Scholar]
- 24.Pöllabauer EM, Pavlova BG, Löw-Baselli A, Fritsch S, Prymula R, Angermayr R, Draxler W, Firth C, Bosman J, Valenta B, et al. Comparison of immunogenicity and safety between two paediatric TBE vaccines. Vaccine 2010; 28:4680-5; PMID:20433803; http://dx.doi.org/ 10.1016/j.vaccine.2010.04.047 [DOI] [PubMed] [Google Scholar]
- 25.Loew-Baselli A, Poellabauer EM, Pavlova BG, Fritsch S, Firth C, Petermann R, Barrett PN, Ehrlich HJ. Prevention of tick-borne encephalitis by FSME-IMMUN vaccines: review of a clinical development programme. Vaccine 2011; 29:7307-19; PMID:21843576; http://dx.doi.org/ 10.1016/j.vaccine.2011.07.089 [DOI] [PubMed] [Google Scholar]
- 26.El‘bert LB, Krasil’nikov IV, Drozdov SG, Grachev VP, Pervikov YV. [Concentrated and purified vaccine against tick-borne encephalitis, prepared by the method of ultra-filtration and chromatography]. Vopr Virusol 1985; 30:90-3. Russian [PubMed] [Google Scholar]
- 27.Heinz FX, Holzmann H, Essl A, Kundi M. Field effectiveness of vaccination against tick-borne encephalitis. Vaccine 2007; 25:7559-67; PMID:17869389; http://dx.doi.org/ 10.1016/j.vaccine.2007.08.024 [DOI] [PubMed] [Google Scholar]
- 28.Romanenko VV, Esiunina MS, Kiliachina AS. [Experience in implementing the mass immunization program against tick-borne encephalitis in the Sverdlovsk Region]. Vopr Virusol 2007; 52:22-5. Russian; PMID:18050713 [PubMed] [Google Scholar]
- 29.Gao X, Nasci R, Liang G. The neglected arboviral infections in mainland China. PLoS Negl Trop Dis 2010; 4:e624; PMID:20436960; http://dx.doi.org/ 10.1371/journal.pntd.0000624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rumyantsev AA, Goncalvez AP, Giel-Moloney M, Catalan J, Liu Y, Gao QS, Almond J, Kleanthous H, Pugachev KV. Single-dose vaccine against tick-borne encephalitis. Proc Natl Acad Sci USA 2013; 110:13103-8; PMID:23858441; http://dx.doi.org/ 10.1073/pnas.1306245110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rumyantsev AA, Chanock RM, Murphy BR, Pletnev AG. Comparison of live and inactivated tick-borne encephalitis virus vaccines for safety, immunogenicity and efficacy in rhesus monkeys. Vaccine 2006; 24:133-43; PMID:16115704; http://dx.doi.org/ 10.1016/j.vaccine.2005.07.067 [DOI] [PubMed] [Google Scholar]
- 32.Schmaljohn C, Custer D, VanderZanden L, Spik K, Rossi C, Bray M. Evaluation of tick-borne encephalitis DNA vaccines in monkeys. Virology 1999; 263:166-74; PMID:10544091; http://dx.doi.org/ 10.1006/viro.1999.9918 [DOI] [PubMed] [Google Scholar]
- 33.Schmaljohn C, Vanderzanden L, Bray M, Custer D, Meyer B, Li D, Rossi C, Fuller D, Fuller J, Haynes J, et al. Naked DNA vaccines expressing the prM and E genes of Russian spring summer encephalitis virus and Central European encephalitis virus protect mice from homologous and heterologous challenge. J Virol 1997; 71:9563-9; PMID:9371620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Süss J. Tick-borne encephalitis 2010: epidemiology, risk areas, and virus strains in Europe and Asia-an overview. Ticks Tick Borne Dis 2011; 2:2-15; PMID:21771531; http://dx.doi.org/ 10.1016/j.ttbdis.2010.10.007 [DOI] [PubMed] [Google Scholar]
- 35.Lundkvist K, Vene S, Golovljova I, Mavtchoutko V, Forsgren M, Kalnina V, Plyusnin A. Characterization of tick-borne encephalitis virus from Latvia: evidence for co-circulation of three distinct subtypes. J Med Virol 2001; 65:730-5; PMID:11745938; http://dx.doi.org/ 10.1002/jmv.2097 [DOI] [PubMed] [Google Scholar]
- 36.Korenberg EI, Kovalevskii YV. Main features of tick-borne encephalitis eco-epidemiology in Russia. Zentralbl Bakteriol 1999; 289:525-39; PMID:10652719; http://dx.doi.org/ 10.1016/S0934-8840(99)80006-1 [DOI] [PubMed] [Google Scholar]
- 37.Jääskeläinen AE, Tikkakoski T, Uzcátegui NY, Alekseev AN, Vaheri A, Vapalahti O. Siberian subtype tickborne encephalitis virus, Finland. Emerg Infect Dis 2006; 12:1568-71; PMID:17176574; http://dx.doi.org/ 10.3201/eid1210.060320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vorob'eva MS, Rasshchepkina MN, Ladyzhenskaia IP. [Vaccines, immunoglobulins, and test systems for the prevention and diagnosis of tick-borne encephalitis]. Vopr Virusol 2007; 52:30-6. Russian; PMID:18050715 [PubMed] [Google Scholar]
- 39.Morozova OV, Bakhvalova VN, Potapova OF, Grishechkin AE, Isaeva EI, Aldarov KV, Klinov DV, Vorovich MF. Evaluation of immune response and protective effect of four vaccines against the tick-borne encephalitis virus. Vaccine 2014; 32:3101-6; In press; PMID:24631082; http://dx.doi.org/ 10.1016/j.vaccine.2014.02.046 [DOI] [PubMed] [Google Scholar]
- 40.Klockmann U, Krivanec K, Stephenson JR, Hilfenhaus J. Protection against European isolates of tick-borne encephalitis virus after vaccination with a new tick-borne encephalitis vaccine. Vaccine 1991; 9:210-2; PMID:2042394; http://dx.doi.org/ 10.1016/0264-410X(91)90157-2 [DOI] [PubMed] [Google Scholar]
- 41.Vorob'eva MS, Rasshchepkina MN, Ladyzhenskaia IP, Gorbunov MA, Pavlova LI, Bektimirov TA. [Comparative study of inactivated cultured vaccines against tick-borne encephalitis manufactured in Russia and in Austria by the “Immuno” firm]. Vopr Virusol 1996; 41:221-4. Russian; PMID:8967069 [PubMed] [Google Scholar]
- 42.Pavlova LI, Gorbunov MA, Vorobjeva MS, Karavanov AS, Grachev VP, Ladyshenskaya IP, Rashchepkina MN, Melnikova LN, Lebedeva TM, Melnikov NA, et al. [Study of tissue-cultured tick-borne encephalitis vaccine, concentrated and inactivated, in the immunization of children and adolescents]. Zh Mikrobiol Epidemiol Immunobiol 1999; 6:50-3. Russian; PMID:10876850 [PubMed] [Google Scholar]
- 43.Chiba N, Osada M, Komoro K, Mizutani T, Kariwa H, Takashima I. Protection against tick-borne encephalitis virus isolated in Japan by active and passive immunization. Vaccine 1999; 17:1532-9; PMID:10195790; http://dx.doi.org/ 10.1016/S0264-410X(98)00360-0 [DOI] [PubMed] [Google Scholar]
- 44.Hayasaka D, Goto A, Yoshii K, Mizutani T, Kariwa H, Takashima I. Evaluation of European tick-borne encephalitis virus vaccine against recent Siberian and far-eastern subtype strains. Vaccine 2001; 19:4774-9; PMID:11535329; http://dx.doi.org/ 10.1016/S0264-410X(01)00218-3 [DOI] [PubMed] [Google Scholar]
- 45.Pavlenko EV, Leonova GN, Krylova NN. [Evaluation of serological methods for studying immune response in persons vaccinated with Encepur Adult tick-borne encephalitis vaccine]. Dal'nevost Zh Infects Patol 2005; 6:29-35. Russian [Google Scholar]
- 46.Prochorova OG, Romanenko VV, Zlobin VI. [Comparative characteristics of immunological activity of tick-borne encephalitis vaccines used during a mass immunization program in the Sverdlovsk region]. Èpidemiol Vaktsinoprofilaktika 2006; 29:33-6. Russian [Google Scholar]
- 47.Leonova GN, Ternovoi VA, Pavlenko EV, Maistrovskaya OS, Protopopova EV, Loktev VB. Evaluation of vaccine Encepur Adult for induction of human neutralizing antibodies against recent Far Eastern subtype strains of tick-borne encephalitis virus. Vaccine 2007; 25:895-901; PMID:17011677; http://dx.doi.org/ 10.1016/j.vaccine.2006.09.014 [DOI] [PubMed] [Google Scholar]
- 48.Leonova GN, Pavlenko EV. Characterization of neutralizing antibodies to Far Eastern of tick-borne encephalitis virus subtype and the antibody avidity for four tick-borne encephalitis vaccines in human. Vaccine 2009; 27:2899-904; PMID:19366574; http://dx.doi.org/ 10.1016/j.vaccine.2009.02.069 [DOI] [PubMed] [Google Scholar]
- 49.Shutova NA, Shkuratova OV, Rouzavina YeV, Vlasova NM, Stavitskaya NKh, Vorobieva MS, Ladyzhenskaya IP, Solyanik RG. [Studing immunologic activity and reactogeneity of “EnceVir®” vaccine during immunization using express-scheme]. Sib Med Zh 2009; 2:30-33. Russian [Google Scholar]
- 50.Pavlenko EV, Leonova GN, Maystrovskaya OS, Krylova NV. [An evaluation of long-term immune response after a complete course of vaccination against tick-borne encephalitis]. Infects Bolezn 2011; 9:39-44. Russian [Google Scholar]
- 51.Orlinger KK, Hofmeister Y, Fritz R, Holzer GW, Falkner FG, Unger B, Loew-Baselli A, Poellabauer EM, Ehrlich HJ, Barrett PN, et al. A tick-borne encephalitis virus vaccine based on the European prototype strain induces broadly reactive cross-neutralizing antibodies in humans. J Infect Dis 2011; 203:1556-64; PMID:21592984; http://dx.doi.org/ 10.1093/infdis/jir122 [DOI] [PubMed] [Google Scholar]
- 52.Ankudinova AV, Romanenko VV, Kovtun OP, Kilyachina AS, Averyanov OYu, Esyunina MS, Shelkova ES. [Results of studying post-vacination immunity in under-3-year-olds vaccinated with TBE-Encepur children]. Immunobiol Mikrobiol Genet 2013; 4:120-3. Russian [Google Scholar]
- 53.Lawson ML, Pham B, Klassen TP, Moher D. Systematic reviews involving complementary and alternative medicine interventions had higher quality of reporting than conventional medicine reviews. J Clin Epidemiol 2005; 58:777-84; PMID:16018912; http://dx.doi.org/ 10.1016/j.jclinepi.2004.08.022 [DOI] [PubMed] [Google Scholar]
- 54.Moher D, Fortin P, Jadad AR, Jüni P, Klassen T, Le Lorier J, Liberati A, Linde K, Penna A. Completeness of reporting of trials published in languages other than English: implications for conduct and reporting of systematic reviews. Lancet 1996; 347:363-6; PMID:8598702; http://dx.doi.org/ 10.1016/S0140-6736(96)90538-3 [DOI] [PubMed] [Google Scholar]
- 55.Juni P, Holenstein F, Sterne J, Bartlett C, Egger M. Direction and impact of language bias in meta-analyses of controlled trials: empirical study. Int J Epidemiol 2002; 31:115-23; PMID:11914306; http://dx.doi.org/ 10.1093/ije/31.1.115 [DOI] [PubMed] [Google Scholar]
- 56.Silber LA, Soloviev VD. Far Eastern tick-borne spring-summer (spring) encephalitis. Am Rev Sov Med 1946; (Spec Suppl):1-80; PMID:21002377 [PubMed] [Google Scholar]
- 57.World Health Organization (WHO) Vaccines against tick-borne encephalitis (TBE). WHO position paper 10 June, 2011. Grading of scientific evidence in support of key recommendations. Table III. Do currently available TBE vaccines protect against clinical TBE with all 3 TBE virus subgroups (European, Far-Eastern, and Siberian)? Available at: http://www.who.int/immunization/TBE_grad_crossprotection.pdf?ua=1 Accessed: April 7, 2014. [Google Scholar]
- 58.Holzmann H, Vorobyova MS, Ladyzhenskaya IP, Ferenczi E, Kundi M, Kunz C, Heinz FX. Molecular epidemiology of tick-borne encephalitis virus: cross-protection between European and Far Eastern subtypes. Vaccine 1992; 10:345-9; PMID:1574920; http://dx.doi.org/ 10.1016/0264-410X(92)90376-U [DOI] [PubMed] [Google Scholar]
- 59.Leonova GN, Pavlenko EV, Maistrovskaya OS, Chausov EV. Protective antibody titer for patients vaccinated against tick-borne encephalitis virus. Proc Vaccinol 2011; 4:84-91; PMID:12590332; http://dx.doi.org/ 10.1016/j.provac.2011.07.01212590332 [DOI] [Google Scholar]
- 60.Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 1997; 25:3389-402; PMID:9254694; http://dx.doi.org/ 10.1093/nar/25.17.3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.The United Nations World Tourism Organization (UNWTO) Tourism Highlights, 2013 Edition. Available at: http://mkt.unwto.org/publication/unwto-tourism-highlights-2013-edition Accessed: April 7, 2014. [Google Scholar]
- 62.Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Ann Intern Med 2001; 135:982-9; PMID:11730399; http://dx.doi.org/ 10.7326/0003-4819-135-11-200112040-00010 [DOI] [PubMed] [Google Scholar]
- 63.Vickers A, Goyal N, Harland R, Rees R. Do certain countries produce only positive results? A systematic review of controlled trials. Control Clin Trials 1998; 19:159-66; PMID:9551280; http://dx.doi.org/ 10.1016/S0197-2456(97)00150-5 [DOI] [PubMed] [Google Scholar]
- 64.World Health Organization (WHO) Vaccines against tick-borne encephalitis (TBE). WHO position paper 10 June, 2011. Grading of scientific evidence in support of key recommendations. Table I: Does a primary series of the currently available TBE vaccines protect children and adults of all ages against clinical TBE for ≥3 years? Available at: http://www.who.int/immunization/TBE_grad_efficacy.pdf Accessed: April 7, 2014. [Google Scholar]
- 65.Chang GJ, Kuno G, Purdy DE, Davis BS. Recent advancement in flavivirus vaccine development. Expert Rev Vaccines 2004; 3:199-220; PMID:15056045; http://dx.doi.org/ 10.1586/14760584.3.2.199 [DOI] [PubMed] [Google Scholar]
- 66.Barrett PN, Schober-Bendixen S, Ehrlich HJ. History of TBE vaccines. Vaccine 2003; 21(Suppl 1):S41-9; PMID:12628813; http://dx.doi.org/ 10.1016/S0264-410X(02)00814-9 [DOI] [PubMed] [Google Scholar]
- 67.Scott P, Moss WJ, Gilani Z, Low N. Measles vaccination in HIV-infected children: systematic review and meta-analysis of safety and immunogenicity. J Infect Dis 2011; 204(Suppl 1):S164-78; PMID:21666158; http://dx.doi.org/ 10.1093/infdis/jir071 [DOI] [PubMed] [Google Scholar]
- 68.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343:d5928; PMID:22008217; http://dx.doi.org/ 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang WWB, Zhi E, Chan ISF. Comparison of methods to analyze coarse immunogenicity data. Proc Joint Stat Meeting, New York. Available at: http://www.amstat.org/sections/srms/Proceedings/y2002/Files/JSM2002-000639.pdf Accessed: April 4, 2014 [Google Scholar]
- 70.The United States Advisory Committee on Immunization Practices (ACIP) Handbook for developing evidence-based recommendations. Version 1.2. Available at: http://www.cdc.gov/vaccines/acip/recs/GRADE/downloads/handbook.pdf Accessed: April 4, 2014. [Google Scholar]
- 71.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005; 5:13; PMID:15840177; http://dx.doi.org/ 10.1186/1471-2288-5-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Beck CR, McKenzie BC, Hashim AB, Harris RC, Zanuzdana A, Agboado G, Orton E, Béchard-Evans L, Morgan G, Stevenson C, et al. Influenza vaccination for immunocompromised patients: systematic review and meta-analysis from a public health policy perspective. PLoS One 2011; 6:e29249; PMID:22216224; http://dx.doi.org/ 10.1371/journal.pone.0029249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33:159-74; PMID:843571; http://dx.doi.org/ 10.2307/2529310 [DOI] [PubMed] [Google Scholar]
- 74.R Development Core Team (2012) R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing, 2012. [Google Scholar]


