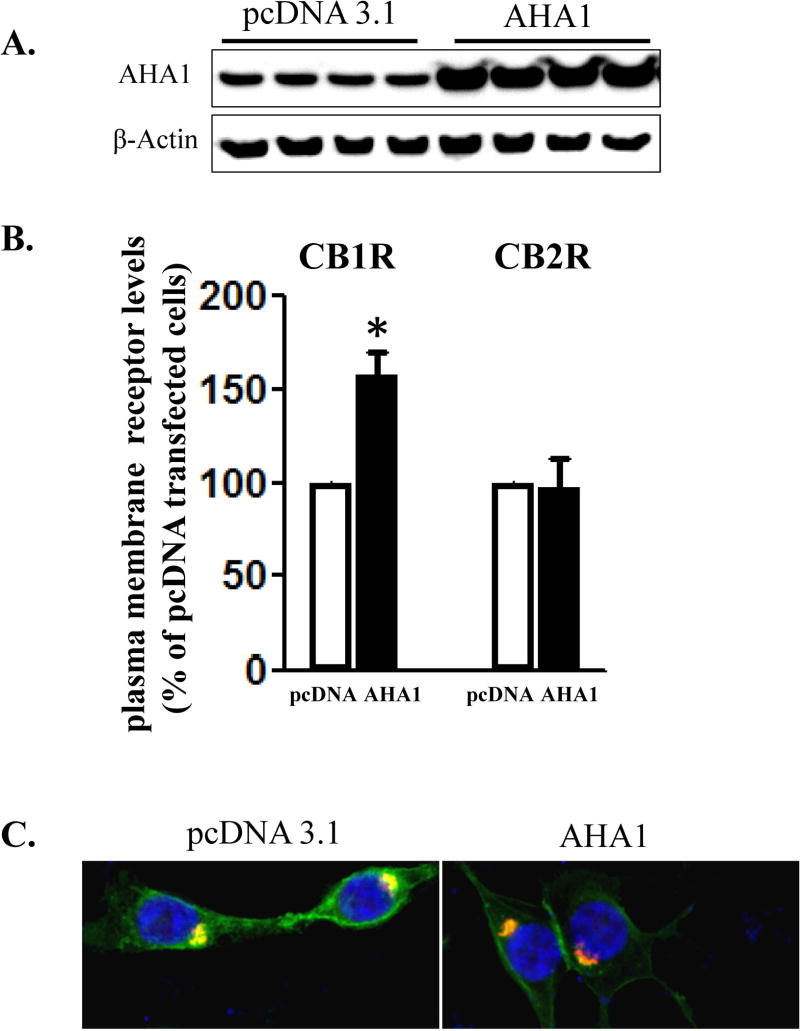
Figure 2. The effects of AHA1 overexpression on the subcellular localization of CB1R and CB2R in HEK293T cells.
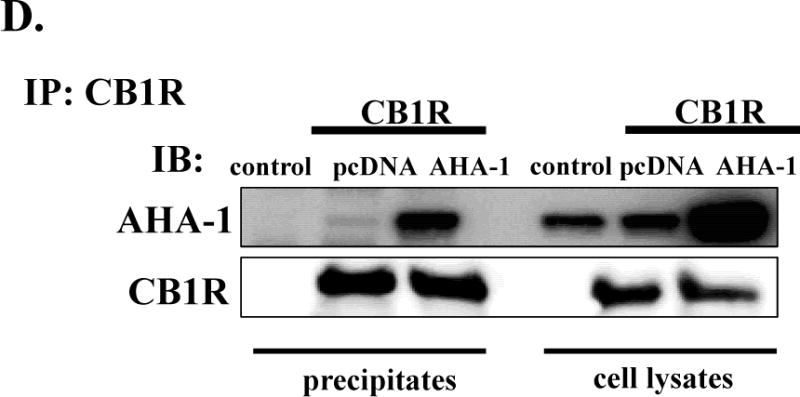
A. AHA-1 levels in control and AHA-1 transfected HEK293T cells. The cells were transfected with pcDNA 3.1 or AHA-1 (2.25 μg/well in 6-well plates) and serum starved for 24 h. Two days after transfection the cells were lysated and 10 μg of total protein were separated by 10% SDS-PAGE and AHA-1 levels were determined by western blot. B. Modulation of CB1R and CB2R plasma membrane levels by AHA1 overexpression. HEK293T were co-transfected with CB1R or CB2R (0.25 μg/well) and pcDNA 3.1 or AHA-1 (2.25 μg/well each) and after six hours the cells were trypsinized and plated on 12-well plates as described in Material and Methods. FBS was withdrawn for 24 h, and two days after transfection the plasma membrane levels of CB1R and CB2R were determined by ELISA as described in Material and Methods. n=12 in each case from three different transfections. *- indicate p < 0.05 compared to pcDNA 3.1 transfected cells. C. Subcellular localization of CB1R in HEK293T cells. The cells were grown on coverslips in 6-well plates and transfected with 3xHA-CB1R (0.1 μg/well) and DsRed-Rab5 (0.1 μg/well). After serum starvation for 24 h, the cells were fixed and permeabilized. The CB1R localization was stained using HA antibody as described in Material and Methods. Blue: DNA staining by 4,6-diamidino-2-phenylindole (nuclear), green: 3xHA-CB1R, red: DsRed-Rab5. The images are representative from three different coverslips, obtained from three independent transfections. D. CB1R interacts with AHA-1 in HEK293T cells. The cells were transiently co-transfected in 10 mm2 dishes with pcDNA 3.1 (10 μg, control), or with CB1R (5 μg) and pcDNA3.1 (5 μg), or CB1R (5 μg) and AHA-1 (5 μg). After two days the cells were solubilized and immunoprecipitated with CB1R antibody as described under Material and Methods. The immunoprecipitates (20 μg/lane) or the lysates (10 μg/lane) were separated by 10 % SDS-Page and subject to western-blotting with AHA-1 and CB1R antibodies. The experiment shown is representative from three independent transfections.