Figure 1.
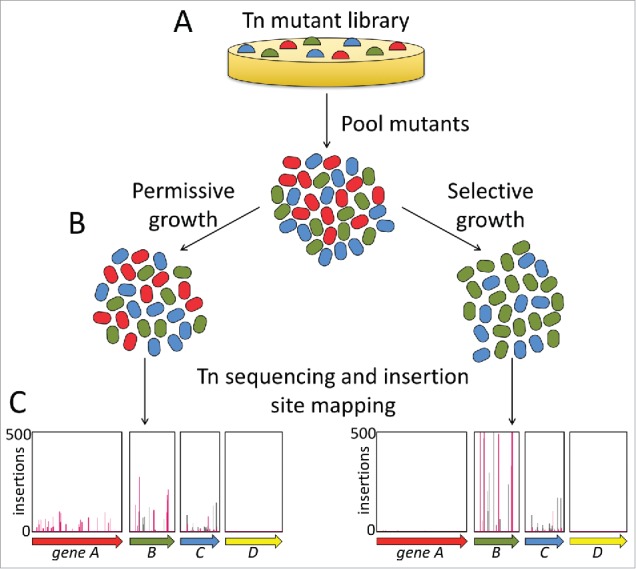
Overview of a typical transposon insertion sequencing fitness-based experiment. (A) A mutant library in the strain of interest is constructed and plated on media permissive to growth. (B) The mutants are pooled and then cultured in permissive and selective conditions, e.g., laboratory media without or with an antimicrobial, respectively. (C) DNA is isolated from the cultured mutant populations and the transposon insertion sites and frequencies are determined using transposon sequencing. A reduction in the frequency of mutants carrying insertions in a particular gene grown under selective conditions compared with permissive conditions indicates that the gene is required for bacterial fitness under the selective condition (e.g., gene A). An increase in the frequency of mutants carrying insertions in a gene grown under selective conditions relative to permissive conditions suggests that there is an advantage to inactivating the gene under the selection used (e.g., gene B). An equal frequency of mutants with insertions in a gene in populations grown under both selective and permissive conditions suggests that the gene does not influence bacterial fitness under the selective condition (e.g., gene C). For some genes (typically around 10% of annotated genes in bacterial genome) there will be no insertions, or very few insertions in the initial mutant pool and after permissive growth (e.g., gene D). These genes are likely to be essential for bacterial survival even under permissive laboratory growth conditions and typically encode housekeeping functions, such as DNA replication. Overall, the information gathered in these experiments allows hypotheses on gene function to be formulated.
