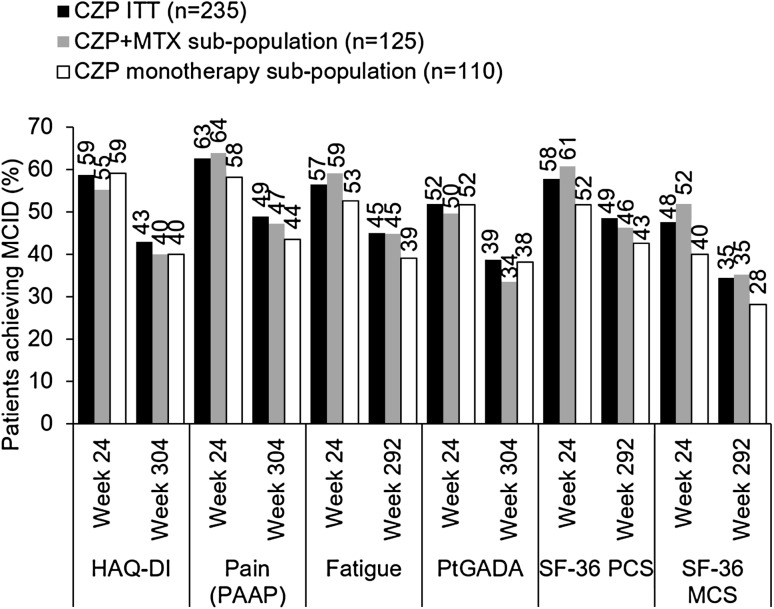
Fig. 4.
Proportion of CZP ITT and CZP monotherapy patients achieving minimal clinically important difference (MCID) in HAQ-DI, pain (PAAP), fatigue, patient global assessment of disease activity (PtGADA), and SF-36 physical (PCS) and mental (MCS) components summary (% of patients, NRI/LOCF). Week 292 data presented where week 304 unavailable. Patients initially assigned to the CZP monotherapy group who achieved an MCID but also received MTX rescue medication prior to each visit were still included in the CZP ITT analysis, but excluded from the CZP monotherapy analysis